A new horizon in rheumatology
By Leonard Calabrese, DO, and Cassandra Calabrese, DO
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
For a generation, those of us in the field of immune-oncology have dreamed of harnessing the power of the immune system to fight cancer. After many failed trials of agents associated with poor outcomes or unacceptable toxicity, the introduction of new immunotherapies for a variety of cancers has energized the field in the last decade.
Currently there are four FDA-approved agents including ipilimumab, nivolumab, pembrolizumab and atezolumab (Table 1). These therapies have produced significant and sometimes dramatic survival benefits in patients with metastatic melanoma, non-small cell lung cancer, renal cell carcinoma, Hodgkin lymphoma and urothelial carcinoma, and are under investigation for many others. In addition, many other agents targeting different sites of immune control pathways are now entering clinical trials.
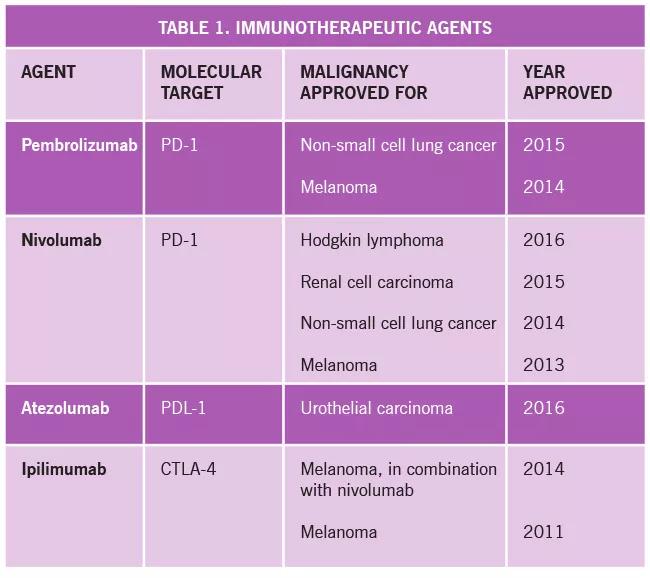
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a307f099-c730-4ac7-b68f-aac3aca4cf02/16-RHE-1747-Calabrese-Inset-Image-Table-01_jpg)
The underlying biology and mechanism of action of these agents exploit the property for the adaptive immune response to restrain itself in situations where a danger signal endures, despite robust effector cell pathway activation. Two situations serve as classic examples of this phenomenon: chronic viral infections such as HIV, where the pathogen may persistently replicate and challenge the host day after day for decades, and malignancies that are perceived as a danger signal but evade host defenses.
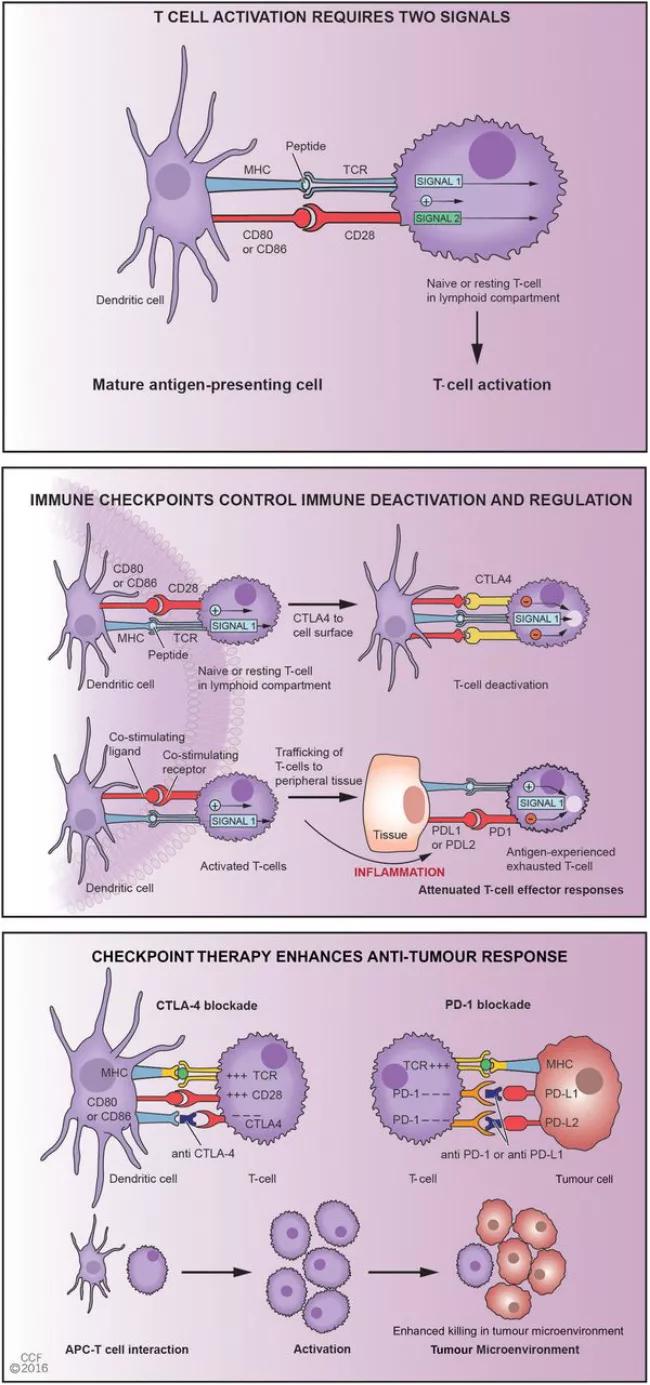
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9cceae73-2d33-4e61-9a8a-f22aca4cc2de/16-RHE-1747-Calabrese-Inset-Image-01_jpg)
Figure 1 summarizes the two pathways that are currently the focus of approved agents.
Recently, a number of complex and challenging rheumatic disorders have been described which add to the more common dermatologic, gastroenterologic, pulmonary and endocrine immune-related adverse events (irAEs). While the exact incidence and prevalence has yet to be described, we estimate rheumatic complications in about five percent of patients. Rheumatic complications have included polyarthritis, vasculitis, PMR, inflammatory myositis, sicca syndrome and more. In a recent analysis of our early experience, we found serious rheumatic complications, the majority requiring glucocorticoids and even a second biologic agent. In almost all cases, these symptoms required interruption of immunotherapy for the underlying cancer and thus are truly life-threatening. We currently lack consensus on optimal therapy.
Advertisement
Vamsidhar Velcheti, MD, of Cleveland Clinic Cancer Center, and I have created a virtual clinic where physicians and advanced practitioners can electronically share and triage patients with irAEs to the appropriate specialist. The clinic also serves as an excellent platform for research.
Dr. L Calabrese is Director of the R.J. Fasenmyer Center for Clinical Immunology in Cleveland Clinic’s Department of Rheumatic and Immunologic Diseases.
Dr. C Calabrese is a fellow in the Department of Rheumatology.
Figure republished with permission from this article in Ann Rheum Dis 2016;0;1-3.
Advertisement
Advertisement

The case for continued vigilance, counseling and antivirals

High fevers, diffuse rashes pointed to an unexpected diagnosis

No-cost learning and CME credit are part of this webcast series

Summit broadens understanding of new therapies and disease management

Program empowers users with PsA to take charge of their mental well being

Nitric oxide plays a key role in vascular physiology

CAR T-cell therapy may offer reason for optimism that those with SLE can experience improvement in quality of life.

Unraveling the TNFA receptor 2/dendritic cell axis