Study clears way for efforts to validate the NIH Toolbox Motor Battery
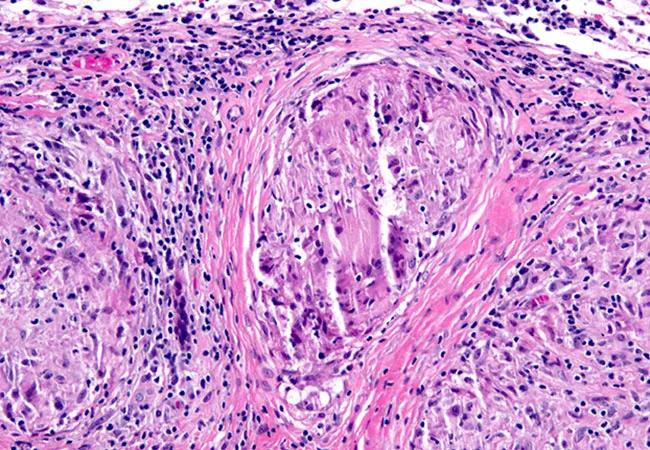
Neurosarcoidosis is second in prevalence only to multiple sclerosis among inflammatory diseases that affect the central nervous system, yet it has eluded effective disease monitoring to date. Researchers led by Cleveland Clinic neurologist Brandon Moss, MD, hope to help change that based on findings from three recent related studies.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Neurosarcoidosis is costly and potentially disabling,” says Dr. Moss, who holds appointments in Cleveland Clinic’s Mellen Center for Multiple Sclerosis as well as the Sarcoidosis Center in Cleveland Clinic’s Respiratory Institute. “While we understand its biology, we lack validated clinical outcome assessment measures to monitor patients’ status.”
Neurosarcoidosis has racial and ethnic variations; it is three times more likely to occur in Black than in white people, although the incidence is relatively high in Scandinavians. It is more common in women than in men. The incidence peaks when people are in their 30s and again in their 50s.
“The lack of validated clinical outcome assessment tools is a barrier to management and follow-up of patients with neurosarcoidosis,” says Dr. Moss. “It’s a barrier to understanding disability in neurosarcoidosis and to tracking disease progression and treatment response.”
To quantify and define shortcomings in clinical outcome assessment measures, Dr. Moss and colleagues have conducted three studies to do the following:
“Our first step was to get input from people who have the disease and can identify the functional domains most relevant to HRQoL,” Dr. Moss explains. He and his colleagues recruited a total of 18 patients from Cleveland Clinic and from Foundation for Sarcoidosis Research (FSR) patient conferences to participate in focus groups. All participants had biopsy-proven sarcoidosis with neurologic complications.
Advertisement
The patients ranged in age from 34 to 61 years. Most (83%) were women; 22% were Black and 72% were white. Participants’ employment status reflected varying levels of disability; 28% worked full time and 50% were disabled.
The focus groups’ concerns reflected a range of neurologic complications of neurosarcoidosis, citing upper and lower extremity function, vision, hearing, cognition, fatigue, pain and depression as key determinants of HRQoL. The groups made a strong case for recognizing heterogeneity and variability in the disease. Findings from the focus groups were presented at a recent meeting of the International Society for Quality of Life Research.
The research team then surveyed 43 physicians from the FSR and the Neurosarcoidosis Consortium Consensus Group. Most participants practiced in the U.S. and worked in centers of excellence. Among the clinics they represented, 21% used clinician-reported outcomes to monitor disease progression and treatment, 21% used institutional performance measures and 30% used patient-reported outcome measures.
Although most survey respondents worked primarily in high-volume sarcoidosis centers, less than half used clinical outcome assessments, most of which were validated for multiple sclerosis and not for neurosarcoidosis.
Survey respondents confirmed that validated clinical outcome assessments are needed to track disease progression and assess treatment response, patients’ needs and HRQoL. They noted, however, that practical barriers include limited time, and lack of validated, disease-specific measures.
Advertisement
Full results of the survey were published online in Multiple Sclerosis and Related Disorders in late 2021.
“We believe that validated measures for neurosarcoidosis may pave the way for better standardization and routine monitoring of impairment,” Dr. Moss observes.
Dr. Moss’s team then evaluated the relevance of the National Institutes of Health (NIH) Toolbox Motor Battery to neurosarcoidosis. The NIH Toolbox, designed to characterize motor function in neurologic diseases, is validated for a variety of neurologic disorders, including multiple sclerosis, another CNS inflammatory disease. The Motor Battery consists of five tests:
“The NIH Toolbox has great potential for assessing functional status measures that are important in neurologic diseases,” notes Dr. Moss, “but the results of testing do not always correspond with specific disorders. When we evaluated the toolbox as a potential measure of neurosarcoidosis impairment, however, we found enough correlation for preliminary validation for its use in neurosarcoidosis.”
The researchers found significant correlations between 9-HPT, 4-MWT and 2-MWT scores and neurological quality-of-life measures. In addition, scores on the 9-HPT, 4-MWT, 2-MWT and select measures of the SBT clearly differentiated functionally dependent and independent patients. The investigators believe that validation of the NIH Toolbox measures for neurosarcoidosis may pave the way for standardization and consistent monitoring of the disease.
Advertisement
Results of this study have not yet been published.
“Improved disability measures in neurosarcoidosis will allow better tracking of disease progression and treatment response in the clinic as well as the research setting, where clinical trials of disease-modifying therapies are lacking,” Dr. Moss concludes. “They will also help us recognize variations among patients — a facet of the disease that concerned patients in our focus groups.”
Image at top: Pathology showing a granuloma in the meninges, the key identifying feature of neurosarcoidosis.
Advertisement
Advertisement

An argument for clarifying the nomenclature

An expert talks through the benefits, limits and unresolved questions of an evolving technology

Recommendations on identifying and managing neurodevelopmental and related challenges

Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits