Research Points to Antibodies as Potential Marker
By Ritu Chakravarti, PhD; Alison Clifford, MD; and Gary S. Hoffman, MD, MS, MACR
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Idiopathic aortitis is an inflammation within the wall of the aorta for which the cause is unknown. It is speculated to be an autoimmune process. Aortitis may occur with or without symptoms, resulting in aneurysm formation or dissection or rupture of the aortic wall, with potentially devastating consequences.
Aortitis may occur either as a focal isolated lesion or as part of a primary systemic large-vessel vasculitis; the most well-known forms of the latter are giant cell arteritis (GCA) and Takayasu’s arteritis (TAK).
Traditionally, GCA, TAK and isolated aortitis have been classified as separate diseases that are distinguished from one another based on age at disease onset (young adulthood in TAK vs. old age [mean age, 74] in GCA) and pattern of vessel involvement (focal in isolated aortitis vs. multifocal in TAK and GCA).
Yet these diseases may be more similar than previously thought. All three subtypes preferentially affect women and share a special predilection for the thoracic aorta. Involvement of large vessels, which is required for diagnosis in TAK, is common in GCA. In GCA, large-vessel disease is found in up to 83 percent of patients at diagnosis by PET imaging and has been documented in 100 percent at postmortem examination. The aortic pathology among subsets is indistinguishable. Glucocorticoid therapy results in remission in GCA and TAK, and relapses are frequent with treatment cessation in both diseases.
The significant overlap observed among focal isolated aortitis, GCA and TAK has raised a number of questions:
Advertisement
• May these separately defined clinical entities actually represent a spectrum within the same disease, and might they share a common etiology?
• If so, could differences be explained by genetic influences or age-related changes in hormone status, tissue protein content and functions, immune function or response to environmental triggers?
Developing an improved understanding of disease pathogenesis and expression of large-vessel vasculitis may lead to identification of safer and more effective therapies. That is the principal goal of our current research.
In collaboration with the Aorta Center in Cleveland Clinic’s Sydell and Arnold Miller Family Heart & Vascular Institute, we have collected more than 120 aorta biopsies (as well as patients’ blood and DNA). So far, we have studied 24 digested tissue samples from aortitis patients and controls.
Although the results are preliminary, we have shown that patients with each of the subclasses of aortitis make antibodies to a protein from digested aorta tissue. Using sophisticated protein analysis techniques (mass spectroscopy), we identified the targeted protein (antigen) to be a member of the 14-3-3 family that has important cell signaling functions in health and disease (see figure).
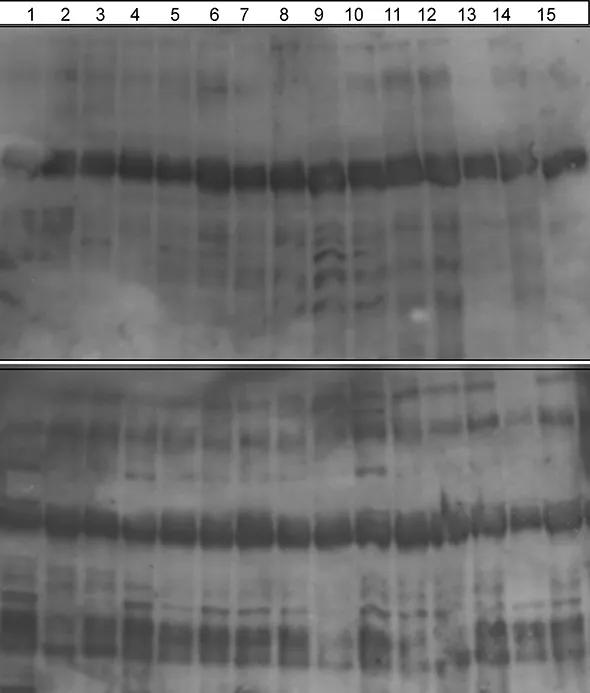
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/0b2ffe01-ed63-4627-ba66-e092bbaaec57/Chakravarti-Inset-590pxl-width_jpg)
Figure. Immunoblots of 15 aorta tissue homogenates: 1 to 9 are controls (noninflammatory aortic aneurysms), and 10 to 15 are different forms of aortitis (Takayasu’s arteritis, giant cell arteritis and focal isolated aortitis). Each Western blot is probed with serum (antibodies) obtained from either control patients (top) or patients with aortitis (bottom) to study whether an autoantigen within the aortic tissue lysates is bound by antibody. Aortitis patient sera react to a 30-kd protein (arrow at bottom) in tissue from both controls and patients with aortitis. However, control sera do not contain antibodies to this protein. Mass spectroscopy was used to characterize the 30-kd protein/antigen, which is in the 14-3-3 family.
Advertisement
We are hopeful that our novel initial finding of anti-14-3-3 antibodies in the serum of aortitis patients may lead to their use as a noninvasive biomarker for diagnosis and disease activity. This finding also adds to a growing body of evidence that these three forms of vasculitis are likely to be part of a shared spectrum of disease. We also are studying these proteins in detail for alterations in composition that may explain why they elicit a pathologic immune response.
Dr. Chakravarti is a project staff member in the Department of Pathobiology, Lerner Research Institute. She can be reached at 216.444.9174 or chakrar@ccf.org.
Dr. Clifford is a vasculitis fellow in the Center for Vasculitis Care and Research, Department of Rheumatic and Immunologic Diseases. She can be reached at cliffoa@ccf.org.
Dr. Hoffman is a staff member in the Center for Vasculitis Care and Research as well as Professor of Medicine, Cleveland Clinic Lerner College of Medicine. He can be reached at 216.445.6996 or hoffmag@ccf.org.
Advertisement
Advertisement

The case for continued vigilance, counseling and antivirals

High fevers, diffuse rashes pointed to an unexpected diagnosis

No-cost learning and CME credit are part of this webcast series

Summit broadens understanding of new therapies and disease management

Program empowers users with PsA to take charge of their mental well being

Nitric oxide plays a key role in vascular physiology

CAR T-cell therapy may offer reason for optimism that those with SLE can experience improvement in quality of life.

Unraveling the TNFA receptor 2/dendritic cell axis