Collaboration studying immunologic perturbations

Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Cleveland Clinic’s Center for Vasculitis Care and Research each year manages more than 500 patients with granulomatosis with polyangiitis (GPA; Wegener’s). This patient volume confers a responsibility to study GPA while also positioning us well to do so. Our efforts in this area include a distinctive ongoing collaboration with Case Western Reserve University (CWRU), profiled below, to study immunologic perturbations in GPA prospectively.
GPA is an uncommon but potentially life-threatening disease characterized by granulomatous inflammation and, typically, medium and small vessel vasculitis. Affected patients experience serious morbidity from effects of the disease itself and the immunosuppressive medications used to treat it. Patients and their physicians share a strong need to better understand this unusual and uncommon disease.
The immunologic underpinnings of GPA are incompletely understood. Anti-neutrophil cytoplasmic antibodies (ANCAs) are observed in GPA, but their role in pathogenesis is controversial. Gaining immunologic insights into GPA will enable better understanding of the mechanisms of disease onset and sustenance and the triggers of relapse. It also will help identify potential therapeutic targets. All of this will allow for better disease management and patient care.
Identification of reliable biomarkers of disease activity is a longstanding need in GPA. Comparing immunologic parameters during active disease and remission ‒ an important focus of our research collaboration ‒ may yield insights into such potential biomarkers.
Advertisement
Our collaboration with CWRU is a prospective observational study funded through Cleveland Clinic’s R.J. Fasenmyer Center for Clinical Immunology. Patients in the study are managed by their own rheumatologists while the research team periodically collects clinical data (captured in an electronic database) paired with blood samples for immunologic studies on a longitudinal basis. The blood samples are sent to the clinical research unit laboratory at Cleveland Clinic, where they are processed for immunologic cells, serum and plasma. These samples are then frozen and stored until they undergo immunologic analyses by two experienced immunology labs at CWRU.
Our interest lies in examining the immune systems of patients with GPA in detail, so we are analyzing T cells, B cells (Figure 1), monocytes and dendritic cells for phenotype, activation, exhaustion and cycling by flow cytometry. In the future, based on preliminary data, we plan to perform functional studies on these cell subsets in the hope of advancing our understanding of their role in disease pathogenesis. We plan to extend these immunologic studies to neutrophils as well, as neutrophils likely play an important role in GPA pathogenesis. One unanswered question is whether immunologic disturbances observed in peripheral blood accurately reflect immunologic perturbations in inflamed tissues. While we are now performing these studies on peripheral blood, we hope to perform relevant studies on tissue samples obtained for clinically indicated reasons in the future.
Advertisement
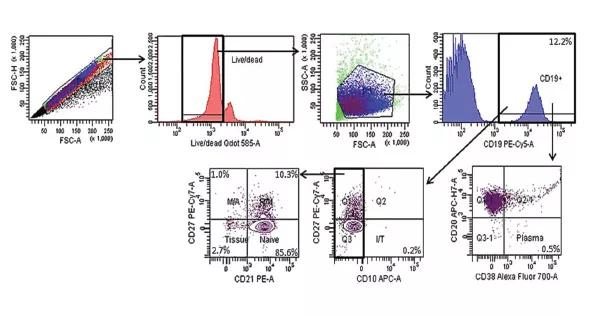
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/755da0ac-81f3-40e8-8ccc-b8a4dd81a085/Khasnis-Inset-Image-590pxl-width_jpg)
Figure 1. B-cell gating strategy for assessment of B-cell subset frequencies in patients with granulomatosis with polyangiitis (GPA; Wegener’s).
The project’s three main goals are:
Advertisement
This project offers a unique opportunity to understand the pathogenesis of GPA. Because this is an observational study and none of its clinical management decisions are influenced by the research protocol, it provides a high level of external validity (i.e., real-world applicability). It also represents an ongoing collaboration between two major academic institutions, Cleveland Clinic and CWRU, that is likely to meaningfully advance our understanding of GPA.
The project’s other key investigators are Leonard Calabrese, DO, and Carol Langford, MD, MHS, of Cleveland Clinic, and Michael Lederman, MD, and Donald Anthony, MD, PhD, of CWRU.
Dr. Khasnis is a staff physician in the Center for Vasculitis Care and Research and the R.J. Fasenmyer Center for Clinical Immunology, both in the Department of Rheumatic and Immunologic Diseases.
Advertisement
Advertisement

A conversation with Leonard Calabrese, DO

The case for continued vigilance, counseling and antivirals

High fevers, diffuse rashes pointed to an unexpected diagnosis

No-cost learning and CME credit are part of this webcast series

Summit broadens understanding of new therapies and disease management

Program empowers users with PsA to take charge of their mental well being

Nitric oxide plays a key role in vascular physiology

CAR T-cell therapy may offer reason for optimism that those with SLE can experience improvement in quality of life.