Response to vaccine in inadequate responders to methotrexate
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
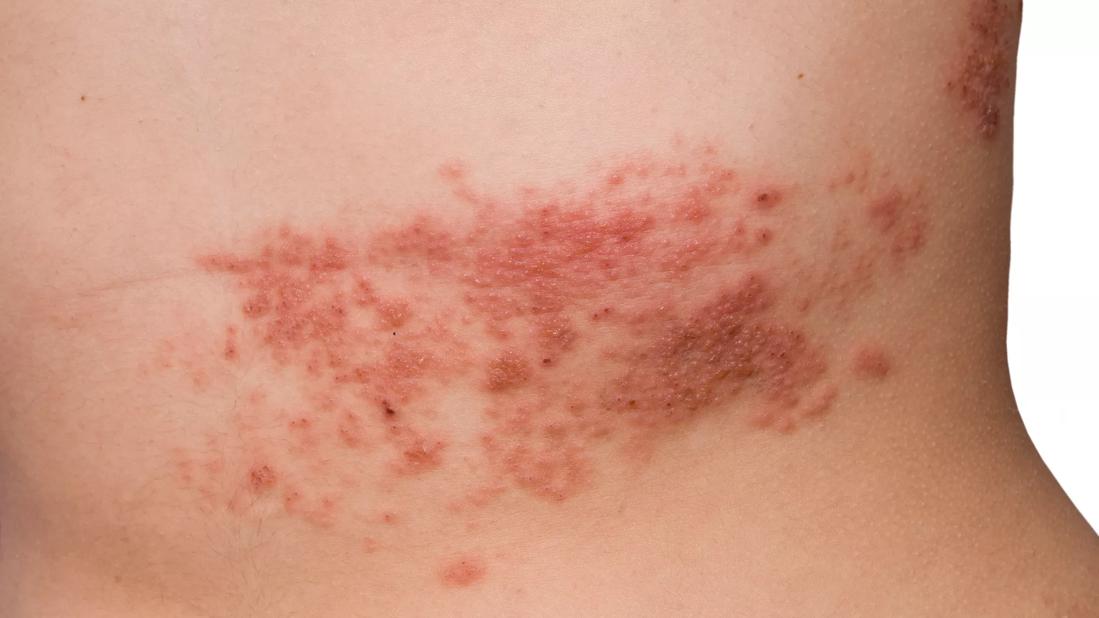
When it comes to herpes zoster (HZ), patients taking immune-modulating biologic therapies are in a double bind. Although they are at heightened risk for zoster due to underlying problems with their integrated immune response, the vaccine against HZ itself poses risks because it is a live attenuated vaccine.
The ORAL Strategy randomized controlled trial evaluates the effect of the live zoster vaccine (LZV) on HZ rates in rheumatoid arthritis (RA) patients who respond inadequately to methotrexate and are treated with biologics. We reported trial results at the 2018 Annual European Congress of Rheumatology.
LZV is highly effective in reducing the incidence of shingles and even better at preventing complications. The CDC’s Advisory Committee on Immunization Practices (ACIP) encourages its use for patients age 60 or older with rheumatic and immunologic diseases even if they are on glucocorticoids in doses up to 20 mg/day and standard doses of nonbiologic immunosuppressants.
In contrast, the ACIP advises that LZV is contraindicated in patients on immune-modulating biologics, due to concerns that these patients could develop a disseminated varicella-like infection from the vaccine’s live virus strain. Yet recent studies suggest that LZV may be safe for patients receiving biologics.
Patients received tofacitinib with or without methotrexate, or adalimumab with methotrexate. Tofacitinib is an oral Janus kinase inhibitor approved for the treatment of RA that unfortunately increases HZ risk. In this phase 3b/4, one-year, triple-dummy active-comparator-controlled trial, patients were randomized to receive tofacitinib 5 mg BID, tofacitinib 5 mg BID plus methotrexate, or subcutaneous adalimumab 40 mg every other week plus methotrexate. In locations where LZV was available, patients ≥ 50 years old received LZV at the investigator’s discretion, 28 days before the first dose of study drug.
Advertisement
Of the patients who received the study drug (N = 1146), 216 received the vaccine 28 days before randomization, and 30 self-reported prior vaccination. The incidence rate (IR) of HZ was similar between the group receiving tofacitinib alone and adalimumab plus methotrexate, and was numerically higher (overlapping CI) in the tofacitinib plus methotrexate group. We found similar incidence rates in patients who received LZV (18.8 percent) and those who did not (81.2 percent).
Overall, LZV was well-tolerated in RA patients who respond inadequately to methotrexate. Unfortunately, we could not assess overall efficacy since less than 20 percent of patients in the study received the vaccine.
Further reducing the impact of the study has been the introduction of SHINGRIX, a recombinant adjuvanted vaccine of far greater efficacy than Zostavax®, which is now recommended by the ACIP as the preferred vaccine. With the growing number of patients on JAK inhibitors such as tofacitinib and baricitinib, prospective studies regarding the efficacy and safety of this vaccine are urgently needed.
Dr. Calabrese is Director of the R.J. Fasenmyer Center for Clinical Immunology in Cleveland Clinic’s Department of Rheumatic and Immunologic Diseases.
Advertisement
Advertisement

A conversation with Leonard Calabrese, DO

The case for continued vigilance, counseling and antivirals

High fevers, diffuse rashes pointed to an unexpected diagnosis

No-cost learning and CME credit are part of this webcast series

Summit broadens understanding of new therapies and disease management

Program empowers users with PsA to take charge of their mental well being

Nitric oxide plays a key role in vascular physiology

CAR T-cell therapy may offer reason for optimism that those with SLE can experience improvement in quality of life.