Trial enrolling now for patients with retinitis pigmentosa caused by RPGR mutation
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/373c5e82-7208-4e89-8450-760f1c353e6c/amd-dna-helix)
Eye with DNA helix
A retinitis pigmentosa (RP) diagnosis once implied unavoidable progressive vision loss. Treatment revolved around low-vision care and mobility training. Genetic testing has changed all that. Now a key component of the patient experience, genetic testing (accompanied by genetic counseling to help interpret test results) has redirected the conversation for patients with RP and other inherited retinal diseases.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“We don’t just talk about RP, but we discuss it as caused by a specific genetic mutation,” says Aleksandra Rachitskaya, MD, a vitreoretinal surgeon at Cleveland Clinic Cole Eye Institute. “Identifying the mutation is important because it can provide insight into how the disease may progress and how other family members may be affected. Perhaps more importantly, it also opens up discussion about gene therapies.”
Currently there is only one gene therapy approved for eye disease by the U.S. Food and Drug Administration. It is for patients with mutations in both copies of the RP65 gene, which can cause RP among other inherited retinal diseases. However, other therapeutics are being studied in multiple ongoing clinical trials, including one multisite trial (NCT03316560) currently recruiting at the Cole Eye Institute for patients with RP caused by RPGR mutation.
The first patient recruited for this RPGR gene therapy trial at the Cole Eye Institute had begun noticing vision problems as a young child, specifically difficulty seeing in dim light. He was diagnosed with X-linked RP due to RPGR mutation. At the time, there was no treatment for him. As an adult, he has been unable to drive and has avoided unfamiliar dark environments.
Classic progression of RP begins with limited night vision, followed by diminishing peripheral vision. As RP progresses, there is loss of central vision, which can lead to legal blindness.
“We prefer to begin treating patients with RP before they start to lose central vision,” says Dr. Rachitskaya, a principal investigator of the RPGR gene therapy trial. “Our patient had problems seeing at night and was losing peripheral vision but still had sufficient central vision, making him a good candidate for gene therapy.”
Advertisement
The goal of gene therapy for inherited retinal disease is not to improve visual acuity, but to stop progression of vision loss and possibly improve functional vision, she notes.
The most common technique is replacement gene therapy, in which a normal gene is introduced into retina cells, where (through transcription and translation mechanisms) protein needed for retinal function is made. If retina cells have sustained too much damage due to advanced disease, the protein produced by gene therapy will be ineffective. That’s why gene therapy is best for patients in earlier stages of disease. Participants in the RPGR gene therapy trial at the Cole Eye Institute must have best corrected visual acuity between 75 letters (20/32) and 35 letters (20/200).
“The measured visual acuity can be quite good in these patients, but it may not adequately reflect their burden of disease,” says Dr. Rachitskaya. “Poor night vision and a restricted visual field can greatly compromise their quality of vision and quality of life.”
In the RPGR gene therapy trial, participants receive one of two doses of an investigational drug. The drug is injected under the retina in the macular region during pars plana vitrectomy. Intraoperative imaging helps the surgeon perform the injection with greater precision, thus improving the safety of the surgery. The patient then receives postoperative care to support healing and control inflammation.
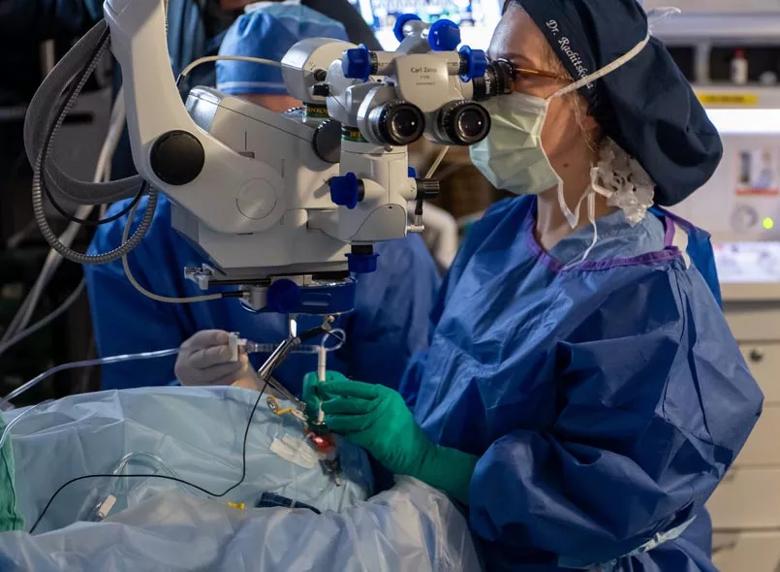
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f93a9b99-94b8-41f2-b4a9-f477a820f417/22-EYE-2668459_CQD-Case-study-involving_Rachitskaya805x590_jpg)
Dr. Rachitskaya delivers investigational gene therapy during pars plana vitrectomy.
Advertisement
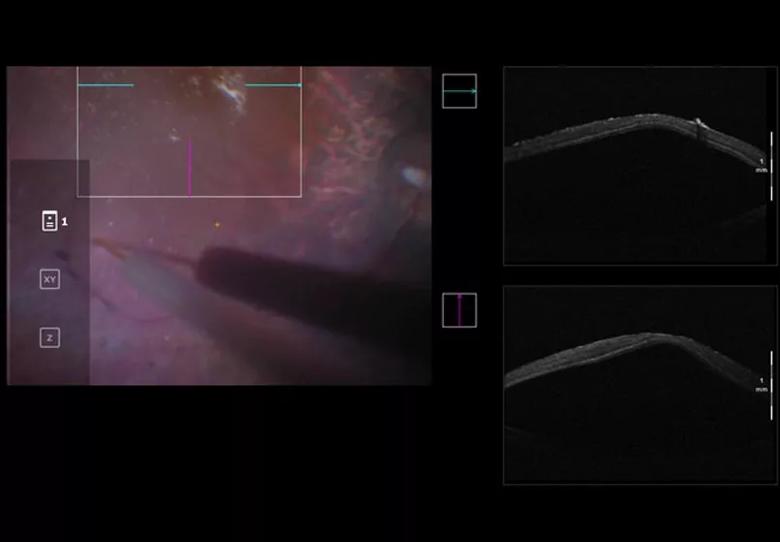
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4dab2358-1681-443a-a809-7ba2272365fb/22-EYE-2668459_CQD-Case-study-involving-805x559-1_jpg)
The test drug is injected under the macula.
Visual acuity is not an appropriate gauge of a patient’s response to this study treatment since best-suited patients have preserved central vision, and the goal of gene therapy is to stop disease progression. Therefore, patients are evaluated both before and after treatment through imaging (such as optical coherence tomography, fundus photography and microperimetry) as well as with two other assessments:
Advertisement
“Patients must understand what each clinical trial involves and be willing to commit to the process,” says Dr. Rachitskaya. “They must understand the potential benefits and risks. We cannot promise that an investigational treatment will benefit a particular patient. That said, each patient’s participation is an important contribution to research and advances the field for every patient with a particular condition.”
Even for patients who do benefit from gene therapy, changes in functional vision can take time. The team at the Cole Eye Institute will follow patients for five years as part of the RPGR gene therapy trial, and then will continue following them after the trial to ensure their long-term eye health.
“There has been a lot of hype about gene therapy for eye disease and, indeed, we have made tremendous strides in this field,” says Dr. Rachitskaya. “However, not all patients are eligible, as genetic testing and a particular degree of disease is required. Providers can help patients better understand the criteria for gene therapy treatments and trials, and set appropriate expectations.”
Advertisement
Advertisement
A review of pathophysiology, risk factors, clinical presentation and treatment options
How computational tools and personalized biomechanics can improve keratoconus detection, ectasia risk assessment and surgical outcomes
Motion-tracking Brillouin microscopy pinpoints corneal weakness in the anterior stroma
Registry data highlight visual gains in patients with legal blindness
Prescribing eye drops is complicated by unknown risk of fetotoxicity and lack of clinical evidence
A look at emerging technology shaping retina surgery
A primer on MIGS methods and devices
7 keys to success for comprehensive ophthalmologists