Lack of appropriate testing remains a barrier for patients eligible for targeted treatment
Two thirds of patients with non-squamous, non-small cell lung cancer (NSCLC) who could be eligible for highly effective targeted treatment are not receiving it, due to a lack of access to testing of the genetic alterations of their tumor. Next-generation sequencing of these tumors would save tens of thousands of life years, with a cost per life year gained of roughly $16,000, according to a new research study.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Due to new targeted treatments, patients with non-squamous NSCLC are living longer, better lives. But that only works if we test those patients’ cancers, identify the genetic alteration and prescribe the appropriate targeted treatment,” says Nathan Pennell, MD, PhD, study co-author and a hematologist/oncologist with Cleveland Clinic’s Taussig Cancer Institute. “We hope this data will stimulate more interest in operationalizing next-generation sequencing.”
29% of patients with non-squamous NSCLC fall into genetic subgroups that could benefit from targeted treatments. There are effective, FDA-approved treatments for nine different genetic mutations of non-squamous NSCLC, yet many institutions are only set up to test for one or two mutations. Next-generation sequencing is a single test that can analyze hundreds of genetic mutations. “If patients don’t receive the appropriate genetic tests at the time of diagnosis to determine eligibility, they end up getting treated with chemotherapy or immune therapy, which has not worked particularly well for this population of patients,” explains Dr. Pennell.
To determine the potential life years that could be saved by broadly implementing next-generation sequencing, the researchers developed a model to evaluate incremental rates of single-gene testing or next-generation sequencing on the basis of life years and cost per life year. Actionable driver oncogenes included for next-generation sequencing were EGFR, ALK, ROS1, BRAF, RET, MET and NTRK. Those for single-gene testing included EGFR and ALK.
Advertisement
Survival distributions were based on published trial averages of median and five-year overall survival, while treatment costs were estimated from drug cost averages. Reimbursement costs were based on data from the Center for Medicare and Medicaid Services.
The researchers discovered next-generation sequencing could make a profound difference. Each incremental 10% increase in next-generation sequencing could results in an average of 2,627.4 additional life years gained, with an average cost savings per life year gained of $75. Replacing single-gene testing at the current rate of 80% with next-generation sequencing would result in an average additional 21,09.6 life years gained, and would reduce the cost per life year gained by an average of $599. If 100% of eligible patients were tested with next-generation sequencing and each identified patient had matched treatment, the total average cost per life year gained would be $16,641.57.
“There’s a misconception that next-generation sequencing is very expensive,” says Dr. Pennell. “We need to separate out the discussion about sequencing for all cancers, where it is still debatable whether everyone should have expensive, broad genetic testing, to lung cancer, where it is indisputable that every patient — specifically those with nonsquamous NSCLC — needs broad testing done,” says Dr. Pennell.
Ten years ago, patients with lung cancer in the US were typically surviving for less than a year. It is now much more common for patients to live many years, but they need the right testing and the right treatment directed towards their cancer. “It’s not a one-size-fits-all situation anymore,” says Dr. Pennell.
Advertisement
Though the study focused on the US population, Dr. Pennell emphasizes the importance of making this testing affordable to everyone worldwide who could benefit from it. Currently, the vast majority of countries lack access to next-generation sequencing and targeted treatment options for NSCLC.
Practitioners looking to adopt next-generation sequencing should talk with the clinician performing the biopsy to ensure they collect a large enough sample, since more tissue is needed for broader mutation testing. The next step would be to speak with their pathology department to ensure they’re working with a vendor who can perform adequate testing in a way that’s fast and easily accessible. There are many people who need to be involved in those conversations, including surgeons, interventional radiologists and pulmonologists to execute and streamline each step in the process. Next-generation sequencing typically takes two the three weeks, so it’s essential that stakeholders work together to develop a system to provide this testing in the shortest time possible.
Advertisement
Advertisement

Treatment assigned FDA review date in June 2025

Cleveland Clinic, the University of Minnesota and University of Cambridge receive $1M grant to develop point-of-care biosensor for early detection and treatment personalization

Hybrid treatment model helps improve cancer care access
Resection, radiotherapy or ablation?

Extent of baseline burden impacts progression-free and overall survival

Quantum computing being studied as a means to help improve predictive performance, accuracy
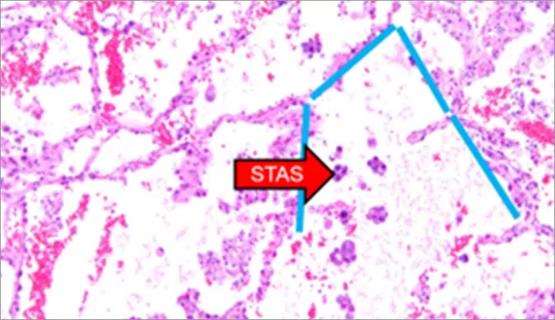
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy

Targeting DNMT1 shows promise in chemotherapy- and immunotherapy-resistant SCLC