Quantitative imaging of white matter integrity beyond what MRI can do
By Mykol Larvie, MD, PhD; Imad Najm, MD; and Yanming Wang, PhD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Positron emission tomography (PET), restricted by nothing less than the cold, hard physical laws governing the decay and detection of positrons, is a highly sensitive and specific imaging modality that is nevertheless limited to spatial resolution on the order of millimeters, typically about 5 mm for a good-quality brain PET scan. For this reason, PET images are much less structurally detailed compared with, for instance, the extraordinarily high resolution that is now routinely achieved with MRI, and thus appear relatively blurry.
Despite this seeming limitation, new PET radiotracers are being developed that provide heretofore unavailable information regarding the smallest possible brain structures — the molecules of which the organ is composed. This ability to evaluate the molecular structure of the brain provides a new approach to mapping brain structure, composition and, ultimately, function.
A multidisciplinary initiative has been launched at Cleveland Clinic to bring to clinical application a PET radiotracer that binds preferentially to the molecular components of myelin, one of the principal components of nerves in the central nervous system (CNS) as well as the peripheral nervous system. This is a new class of imaging agent that has never before been used in humans and represents the first agent of its kind to be brought to clinical application.
This compound is known by its chemical name, [N-methyl-11C]-4,4′-diaminostilbene (MeDAS), now termed Myelivid. The molecule was developed by a group at Case Western Reserve University in Cleveland, and the translational research program for use in human patients is being led by faculty from Cleveland Clinic’s Neurological and Imaging Institutes.
Advertisement
Disorders of myelination may reflect an intrinsic defect, most commonly associated with specific genetic conditions characterized as dysmyelinating diseases, as the myelin is abnormally formed when it is initially expressed. Alternatively, myelin that is normally formed in development may be perturbed in acquired myelin disorders known as demyelinating diseases, the best known of which is multiple sclerosis (MS). Additionally, as we have discovered in research related to the development of myelin PET, there is disruption of myelin in many other brain diseases not typically known for their effects on myelin, such as epilepsy, stroke, neurodegenerative disease and possibly neuropsychiatric disease. Consequently, characterization of the state of myelination in the brain provides a fundamental measure of brain integrity.
Conventional approaches to evaluation of white matter in the brain and spinal cord rely on MRI, particularly T1- and T2-weighted images and diffusion imaging. Structural T1- and T2-weighted images are dominated by the water content in white matter, which is a nonspecific parameter that relates only in part to the integrity of myelin. Diffusion imaging can be used to model white matter tracts with techniques known as diffusion tensor imaging (DTI). A limitation of DTI methods is that the diffusion tensor signal arises principally from water within the axons and thus provides limited information regarding myelin integrity. While this area requires further investigation, including myelin PET analysis, there is evidence that substantial myelin deficits may be relatively inapparent by conventional MRI methods.
Advertisement
Recognizing the lack of PET radiotracers and limitations of MRI for evaluation of brain white matter, Professor Yanming Wang led a team in the development of a small-molecule radiotracer with selective binding to myelin. To function effectively as a brain imaging agent, the tracer had to meet several stringent molecular design criteria:
The result of this drug discovery effort is the molecule MeDAS,1 now known as Myelivid (Figure 1).
![Molecular structure of Myelivid ([N-methyl-11C]-4,4′-diaminostilbene, MeDAS)](https://assets.clevelandclinic.org/transform/309ac521-ff64-4179-acfb-0b1ec2543a20/16-NEU-594-Larvie-Myelin-PET-inset1_jpg?io=transform:fit,width:780)
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/309ac521-ff64-4179-acfb-0b1ec2543a20/16-NEU-594-Larvie-Myelin-PET-inset1_jpg)
Figure 1. Molecular structure of Myelivid ([N-methyl-11C]-4,4′-diaminostilbene, MeDAS).
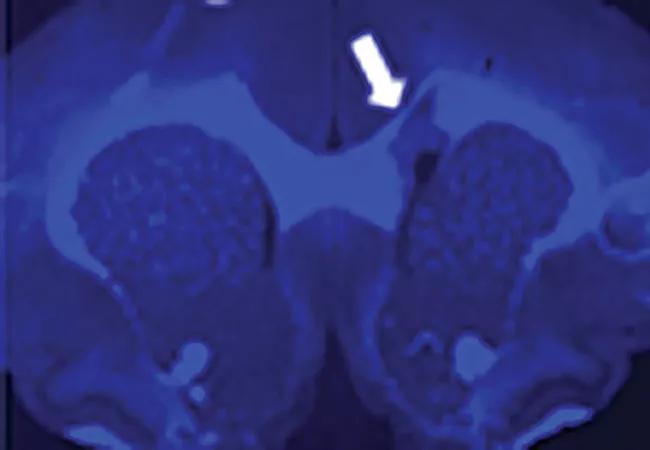
To assess its translational potential for use in humans, Myelivid underwent extensive preclinical testing.1,2 This included tests in experimental systems to demonstrate that the molecule specifically detected myelin in normal brain tissue and could sensitively demonstrate abnormal myelin changes as well (Figure 2).
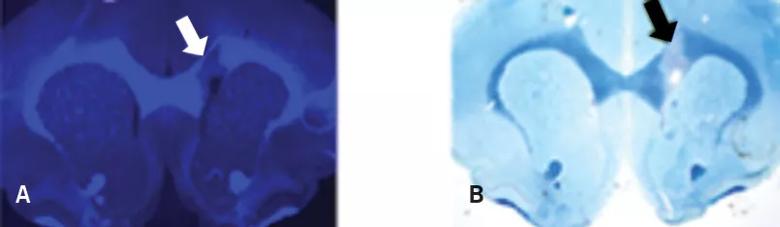
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/8aba2bd5-58ca-4d96-b973-730d8d5b5369/16-NEU-594-Larvie-Myelin-PET-inset2_jpg)
Figure 2. Myelivid stains normal myelin and sensitively detects myelin lesions. Brain tissue specimens from a murine model were stained with Myelivid (A) and Luxol fast blue stain (B) and visualized with fluorescence microscopy (A) and light microscopy (B). The bright signal in panel A is due to intrinsic fluorescence properties of Myelivid. Stains were performed on adjacent slices of brain tissue obtained from the same subject. Arrows indicate the site of a myelin lesion, which is conspicuous due to decreased uptake of Myelivid and Luxol fast blue stain.
Advertisement
Testing was then performed to ensure that the molecule could serve reliably as a radiotracer, which required testing radiolabeled Myelivid in brain tissue specimens (Figure 3). Having established that the molecule is a sensitive and specific radiotracer, extensive testing was done to evaluate its uptake in the intended tissues of the CNS (Figure 4).
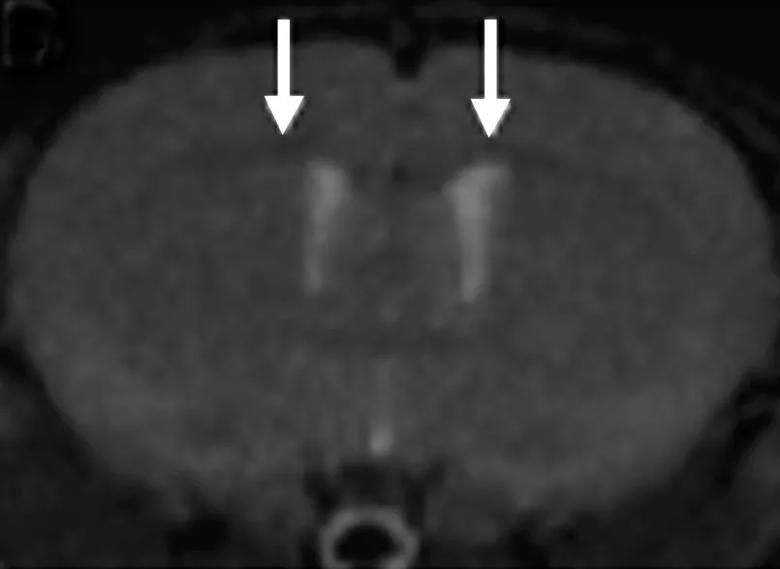
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/670dea63-f3cf-4dff-b65b-b7b89061ff8b/16-NEU-594-Larvie-Myelin-PET-inset3_jpg)
Figure 3. Myelivid is a sensitive radiotracer. Brain tissue from a murine model was stained with radiolabeled Myelivid and detected using autoradiography. Arrows indicate myelin lesions, which show up as bright spots in the autoradiograph due to decreased Myelivid uptake in these areas.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f1bee54d-2243-49fa-8048-3ab69efbb853/16-NEU-594-Larvie-Myelin-PET-inset4_jpg)
Figure 4. Whole-body PET images using Myelivid radiotracer in a murine model system. The brain and spinal cord are clearly defined here by Myelivid uptake. Additional radiotracer uptake is identified in other parts of the body, reflecting both specific and nonspecific binding.
Structural brain imaging is currently dominated by MRI, which is capable of revealing brain tissue with extraordinary precision and detail. However, MRI has fundamental limitations that have not been overcome with advances in technique such as diffusion-weighted imaging and DTI. These include the inability to distinguish tissues of different biologic structure if the chemical composition is similar, as well as the qualitative nature of MRI.
Myelin PET with Myelivid recognizes specific molecular structures in myelin, and therefore provides sensitive detection of intact myelin, whereas MRI cannot distinguish between the molecular structures but is most sensitive to myelin’s water content, which may not accurately reflect the biological integrity of the tissue. Additionally, since PET is based on the detection of radionuclide decay events, Myelivid PET provides an intrinsically quantitative measure of myelin integrity and quantity in the brain, which can be calibrated and used for detecting changes over time in a single patient or for comparing the abundance of myelin in different patients. This enables detection of subtle changes in a patient that may reflect response to therapy, for instance. Additionally, measurement of the absolute quantity of myelin in brain tissue may allow thresholds to be determined for diagnosing specific disease states.
Advertisement
An additional advantage of PET imaging is that implanted electronic devices can be safely accommodated in a PET scanner, and metallic hardware generally does not strongly interfere with brain imaging. Consequently, patients with devices such as vagal nerve stimulators or EEG electrodes — commonly used in patients with epilepsy, for instance — can be safely and reliably imaged with Myelivid PET.
Check out this companion post that profiles the clinical applications of Myelivid PET that Cleveland Clinic has undertaken to date along with a look ahead to additional clinical applications being planned.
Advertisement

Recommendations on identifying and managing neurodevelopmental and related challenges

Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

An expert’s take on evolving challenges, treatments and responsibilities through early adulthood

Comorbidities and medical complexity underlie far more deaths than SUDEP does