Initial studies show promise in localizing elusive epileptic foci
By Mykol Larvie, MD, PhD; Imad Najm, MD; and Yanming Wang, PhD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Although positron emission tomography (PET) images are much less structurally detailed than their MRI counterparts, new PET radiotracers are being developed that provide unprecedented abilities to evaluate the molecular structure of the brain.
A multidisciplinary initiative has been launched at Cleveland Clinic to bring to clinical application a novel PET radiotracer called MeDAS (Myelivid)1 that binds preferentially to the molecular components of myelin. This is a new class of imaging agent that has never before been used in humans and represents the first agent of its kind to be brought to clinical application.
We developed a companion Consult QD post that outlines the development of Myelivid and the many advantages that myelin PET offers over MRI for selected brain imaging applications. Here we expand on that initial post by profiling the clinical applications of Myelivid that Cleveland Clinic’s Neurological and Imaging Institutes have underway currently (in epilepsy) and have in the works in additional neurological conditions.
One of the major goals of the presurgical evaluation of patients with medically intractable (pharmacoresistant) focal epilepsy is localization of the epileptic focus. This constitutes the most important determinant of seizure freedom following potential surgical resection, particularly in patients with epilepsy due to focal cortical dysplasia (FCD). Despite advancements in MR technology, MRI studies remain negative in almost 25 percent of these patients.
Advertisement
To improve lesion detection in patients with MRI-negative epilepsies, we recently started to use Myelivid on human FCD tissue and in animal models of cortical dysplasia. Our preliminary data show significant and focal changes in the imaged myelin in the dysplastic human tissue and the dysplastic animal brains. Figure 1 shows the difference in myelin in normal and abnormal brain tissue using conventional myelin stains, while Figure 2 shows that these differences are similarly depicted with Myelivid staining.
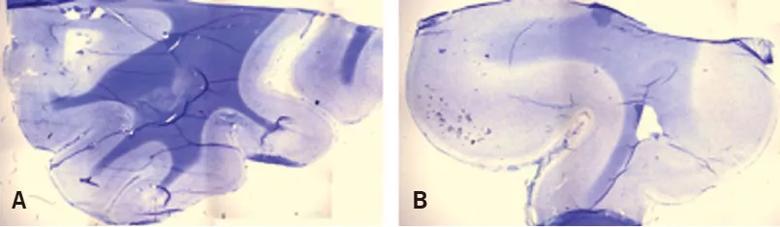
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b15fba5f-d94c-49e5-9882-f0db2b245f46/18-NEU-595-Larvie-Myelin-PET-inset1_jpg)
Figure 1. Myelin is substantially depleted in regions of some focal cortical dysplasias. Brain specimens from normal human brain tissue (A) and abnormal human brain tissue in the region of a focal cortical dysplasia (B) were stained with myelin stains Luxol fast blue and cresyl violet acetate and visualized with light microscopy. Normal brain tissue (A) shows strong uptake of stain in white matter compared to cortex, as well as a sharp margin between white matter and gray matter. The abnormal brain tissue (B) shows markedly decreased volume of white matter compared to gray matter, abnormally thickened cortex representing the focal cortical dysplasia, poor definition of the gray matter/white matter boundary and sharply decreased stain uptake within the white matter, reflecting myelin depletion.
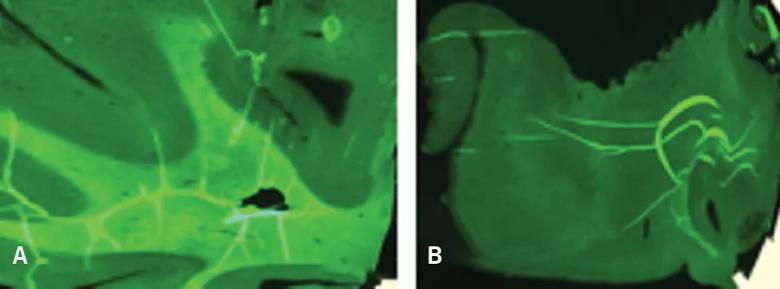
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/44f0df6e-3011-4e63-b1bf-afd14fdc178d/18-NEU-595-Larvie-Myelin-PET-inset2_jpg)
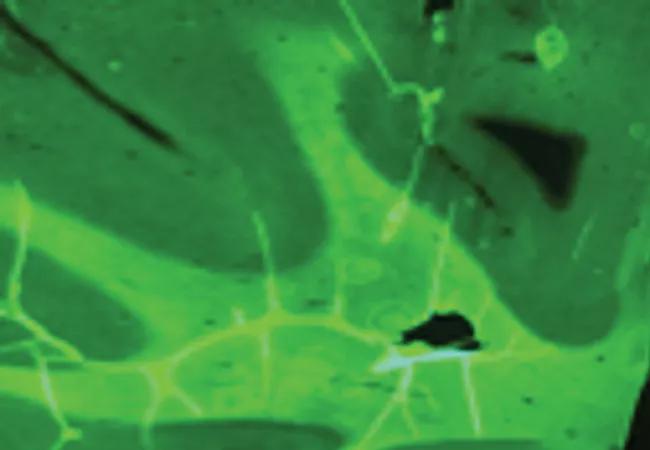
Figure 2. Myelivid detects normal and abnormal myelin in human brain tissue. Brain specimens stained with Myelivid were visualized with fluorescence microscopy. The patterns of normal and abnormal myelin seen in Figure 5 using conventional myelin stains are similarly seen using Myelivid. Normal human brain tissue (A) shows robust Myelivid uptake and a sharp boundary between gray and white matter. Abnormal human brain tissue in the region of a focal cortical dysplasia (B) shows very low-level Myelivid uptake, reflecting markedly depleted myelin, and poor definition of the gray matter/white matter boundary.
Advertisement
We will continue to study the myelin changes in FCD and possibly other epileptic lesions. As a second step, we will be starting a first-in-human trial to study the usefulness of Myelivid in patients with pharmacoresistant epilepsy undergoing presurgical evaluation at Cleveland Clinic’s Epilepsy Center. Our ultimate goal is to use this exciting new imaging method to improve lesion identification and thus provide new opportunities for intervention and optimizing surgical outcomes.
Myelivid PET provides a new approach to quantitative imaging of brain microstructural integrity, providing information not available from other PET radiotracers or existing MRI methods. This presents the opportunity for widespread application in neurologic disease, and we expect to give priority to the following areas in our initial clinical trials with Myelivid PET.
Multiple sclerosis (MS). MS is the most common of the demyelinating diseases, and the clinical and research programs of Cleveland Clinic’s Mellen Center for Multiple Sclerosis are among the largest in the world. In collaboration with Robert Fox, MD, a Mellen Center neurologist and Vice Chair for Research in the Neurological Institute, we will study Myelivid PET in patients with a variety of MS phenotypes.
As MS can affect myelin in multiple CNS compartments, including the brain, spinal cord and optic nerves, we will test Myelivid to determine its ability to detect and characterize demyelinating lesions in all these areas. The quantitative results may be useful for detecting improvement or worsening of disease, and thus may help determine optimal therapy for individual patients. Similarly, Myelivid PET may be useful in the design, development and evaluation of new therapies to treat MS and other demyelinating diseases.
Advertisement
Stroke rehabilitation. Ischemic stroke injures the brain directly through deprivation of oxygen and nutrients and removal of waste from brain tissue. Additionally, brain tissue is damaged indirectly due to disconnection of remote brain areas that continue to receive adequate blood flow but no longer receive synaptic input from the ischemic brain tissue. This injury, known as diaschisis, is mediated by connectivity of white matter tracts and may contribute significantly to the deficits experienced by stroke victims.
Andre Machado, MD, PhD, and Kenneth Baker, PhD, of Cleveland Clinic’s Neurological Institute and Lerner Research Institute have pioneered a program of stroke rehabilitation based on cerebellar stimulation2 in an attempt to reverse the effects of crossed cerebellar diaschisis to aid in recovery from stroke. Myelivid PET will be tested in stroke patients to study the degree of white matter loss following acute infarction and the degree to which white matter recovery correlates with improved function following stroke rehabilitation. This will provide a quantitative metric to permit better evaluation of interventions to improve recovery following stroke, which could sharply accelerate development of new stroke rehabilitation therapies.
Spinal cord injury. Spinal cord injury causes disruption of long, myelinated axons connecting the brain with peripheral nerves. Repair of myelinated spinal cord axons can be sensitively assessed with Myelivid PET3 and used for the evaluation and development of diagnostic and therapeutic approaches to spinal cord injury.
Advertisement
Advertisement

Recommendations on identifying and managing neurodevelopmental and related challenges

Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

An expert’s take on evolving challenges, treatments and responsibilities through early adulthood

Comorbidities and medical complexity underlie far more deaths than SUDEP does