New technologies and tools offer hope for fuller understanding
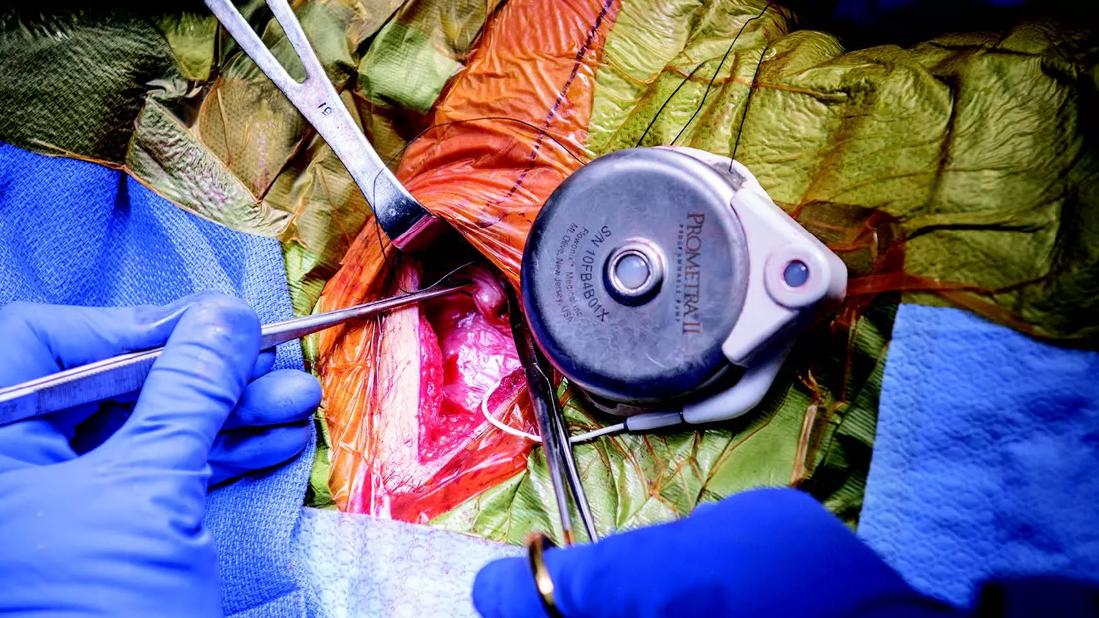
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6a25819e-456e-4031-8998-e558cd0cc59f/Pain-Pump-Image_jpg)
Pain pump implant
Long before the introduction of the first neuromodulation device in the 1960s, human beings were harnessing the pain-relieving power of electrical current through less technical means: fish.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“It actually started with my ancestors, the ancient Egyptians,” says Cleveland Clinic pain management specialist Nagy Mekhail, MD, PhD. “The priests used to take the limb of a patient and put it in a pool filled with torpedo fish or electric eels because it helped with the pain, which is in essence what we do today with spinal cord stimulation. We put electric energy in the area of the spinal cord that corresponds to the painful part of the body, and it relieves pain.”
But despite technical advances that have allowed surgeons to implant pain-relieving devices in the human body, says Dr. Mekhail, modern neurostimulators have one thing in common with the electric eel method: Science still cannot fully explain how they work.
A more precise understanding of the mechanism of spinal cord stimulation (SCS) is the first of what Dr. Mekhail says are two unmet needs in the field of neuromodulation. Meeting those needs has the potential to radically improve care for patients with intractable pain.
“There are studies that claim we stimulate the dorsal column or the dorsal horn, but there is no proof that we are stimulating a specific target of the spinal cord,” he says. “And even if, as some of these studies claim, we stimulate a specific target, we don’t know how much of the dose is going to the target or the response of the target.”
That missing information may account for why as many as 22% of high-frequency devices are removed within two years for lack of sufficient therapeutic effect, he says. “I’m not against this treatment. It is effective in some patients, and in some cases it is lifesaving. But it’s an expensive device that is implanted in the patient’s body,” says Dr. Mekhail. “We need to understand the mechanism of action.”
Advertisement
Until recently, he adds, advancement in SCS devices made incremental improvements without answering the biggest question. With the arrival of closed-loop SCS in the form of the recently approved device Evoke, he sees hope for meaningful improvements.
“Closed-loop stimulation presents our first opportunity to monitor and record specific neurophysiologic functions of the spine,” he says.
Evoke can measure the action potential of the neuron stimuli, the conduction velocity of specific nerves, and the reactivity and the excitability of certain nerves. This device sends action potential data to its generator, then adjusts the next wave of a stimulation to produce the same impulse. This interactive stimulation takes place in real time.
“If the frequency is 40 per second, Evoke makes 40 adjustments per second. It makes close to 3.5 million adjustments a day,” Dr. Mekhail says. “We know exactly which fibers of the spinal cord we are stimulating, and that is reflected in better outcomes.” In the first two years of studies with Evoke, none was removed for lack of therapeutic benefit.
Among some pain management specialists, targeted drug delivery (TDD) via intrathecal pump is often regarded as a last-ditch option for patients whose pain has not been controlled with other methods, including SCS. Better evidence is needed, says Dr. Mekhail, to decide whether a given patient will best respond to SCS or TDD.
“Since we started using these two modalities, we have not had a scientific way of identifying which patient should receive SCS and which should get the pump,” he says. “We have followed what we call the conventional wisdom.”
Advertisement
Electrical stimulation of the spine has been assumed to be safer, and it is easily adjusted; patients can turn it off if a problem arises. The pump delivers opioids and anesthetics directly to the spine. While direct delivery allows for effective pain relief at doses lower than conventionally delivered drugs, concerns about ongoing use of opioids have remained.
“There has been a feeling that SCS was safer, but that was really based on feelings, not on data,” Dr. Mekhail says.
A Cleveland Clinic study published in 2020 addressed this by using retrospective data from the health system’s pain management program to examine two cohorts: patients whose nonmalignant pain was successfully treated with SCS, and those who pursued TDD after failure with SCS. Of the 945 cases reviewed, 119 achieved adequate pain relief with TDD after failure with SCS. The characteristics of those who experienced success with TDD differ in several ways:
“We were able to come up with a formula using data from the patient’s history, including sex, age, diagnosis, whether they have neuropathic pain, postlaminectomy syndrome, spine-related pain, and if they have depression,” Dr. Mekhail says. “We plug the data into the formula and, if the patient scores above a certain limit, SCS is good; below that limit, targeted drug therapy is recommended.”
Advertisement
Cross-validation confirmed that the predictive tool had a 95% accuracy rate, but the original research was limited by two factors: It relied on data from a single institution, and it did not include patients who had started with TDD and moved to SCS.
The researchers then worked with Medtronic to attempt to validate the formula through a prospective study using data from patients from across the country. Six-month results yielded a similarly high reliability rate.
“Had the physicians applied this formula before choosing the technology, they might have saved money and done fewer procedures,” he says. “If we apply the formula, I think we’ll be better off, but the formula is not the bible. We may find factors that we missed, or find that the results were not statistically significant.”
Still, he says, a predictive formula provides a good start for identifying the best path for patients based on science. The decision of which modality to recommend has historically focused on identifying those patients for whom a select therapy should not be offered rather than on those who will best benefit from a given treatment, says Dr. Mekhail. Presurgical identification of patients who are likely to experience long-term pain relief with a given neuromodulation device remains a “holy grail” of pain management practice.
Advertisement
Advertisement
Researchers seek solutions to siloed care, missed diagnoses and limited access to trauma-informed therapies
Cleveland Clinic study investigated standard regimen
Researchers identify the neurologic evolution of pain-related learning
Tapping into motivational interviewing to guide behavioral change
Self-efficacy mindset, burst therapy and increased biofeedback may help improve outcomes
How functional restoration can help children with these conditions marked by unexplained pain with stigmatized symptoms
Two-hour training helps patients expand skills that return a sense of control
National Institutes of Health grant supports Cleveland Clinic study of first mechanism-guided therapy for CRPS