A primer on sustained release options
By Rishi P. Singh, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The retina community wants and needs more durable treatment options for retinal vascular disease. In the 2023 Preferences and Trends Survey conducted by the American Society of Retina Specialists, when asked “What potential benefits of anti-VEGF lasting longer in the retina are most important to you?” 81.1% of U.S. respondents selected “Longer maintenance of drying effect,” and 81.4% selected “Better long-term vision outcomes.”
Newer drugs certainly have increased treatment durability. This table shows three therapies recently approved by the U.S. Food and Drug Administration (FDA).
| Aflibercept | Faricimab | Ranibizumab port delivery system | |
|---|---|---|---|
| FDA-approved indications | Neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME), diabetic retinopathy | nAMD, DME, retinal vein occlusion | nAMD |
| Clinical dose | 8 mg | 6 mg | 2 mg |
| Dosing interval for nAMD | Loading dose of 3 injections at 4-week intervals and then every 8-16 weeks | Loading dose of 4 injections at 4-week intervals and then every 8-16 weeks | Every 24 weeks |
| FDA-approved indications | |||
| Aflibercept | |||
| Neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME), diabetic retinopathy | |||
| Faricimab | |||
| nAMD, DME, retinal vein occlusion | |||
| Ranibizumab port delivery system | |||
| nAMD | |||
| Clinical dose | |||
| Aflibercept | |||
| 8 mg | |||
| Faricimab | |||
| 6 mg | |||
| Ranibizumab port delivery system | |||
| 2 mg | |||
| Dosing interval for nAMD | |||
| Aflibercept | |||
| Loading dose of 3 injections at 4-week intervals and then every 8-16 weeks | |||
| Faricimab | |||
| Loading dose of 4 injections at 4-week intervals and then every 8-16 weeks | |||
| Ranibizumab port delivery system | |||
| Every 24 weeks |
In the PULSAR study, 78% of patients on high-dose aflibercept for nAMD were able to maintain dosing at 16-week intervals. Fifty-three percent (53%) achieved 20-week intervals.
In the TENAYA and LUCERNE trial, at two years, 67% of patients on faricimab for nAMD were able to maintain dosing at 16-week intervals. More than 80% were able to maintain dosing at intervals of 12 weeks or longer.
Clearly, ophthalmology practices have some good treatment options right now. However, we potentially need even longer treatment durability for our patients.
The biggest reason treatment fails in patients is the inconvenience of continuing injections. For example, in my clinic at Cleveland Clinic Florida, I take care of snowbirds. These patients want to come back for treatment, but they’re interrupted when they leave Florida and live elsewhere for several months of the year.
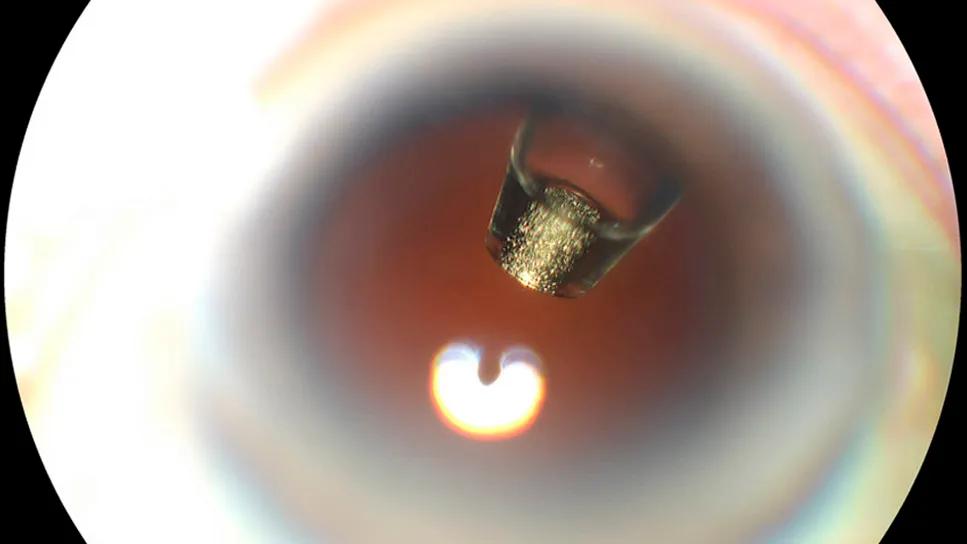
The ranibizumab port delivery system (shown inside the eye of a patient, above) was originally approved after the phase 3 Archway study. Archway was an open-label, randomized clinical trial involving patients previously treated for nAMD. The primary endpoint was the change in best-corrected visual acuity (BCVA), which the treatment did achieve. And, the center point thickness of the retina didn’t change a bit when compared with monthly ranibizumab injection.
Advertisement
However, there were some adverse events, including vitreous hemorrhage within the first postoperative period. Then there were some major issues with the delivery mechanism, including septal dislodgement due to placing the needle improperly or pressing too hard on the septum. A 2022 report showed septal dislodgement in 33 out of 1,400 (2.3%) implants. In the real world, it was more like 5.3% — a significant number of patients. The device was voluntarily recalled by the manufacturer.
The issue was related to the design of the septum and the refill needle. A lot of force was required to get through the septum, and retina specialists had to use a microscope to do it rather than using loupes.
Today, clinical trials of the ranibizumab port delivery system have resumed with a newly designed implant featuring a different septum overmold. Hopefully, this new design will be more stable.
The refill exchange needle also has been updated. A study reported that while the needle with the old implant had about a 47% chance of making 21 punctures, the updated needle has a 52% chance of making up to 36 punctures.
Other drugs that are showing promise in clinical trials and for use in practice are tyrosine kinase inhibitors. These drugs, such as vorolanib and axitinib, essentially affect the intracellular downstream effects of both angiopoietin receptors and VEGF receptors.
Vorolanib has been studied in several early phase clinical trials. For example, EYP-1901 delivers vorolanib through a bioerodible insert similar to the approach used with the fluocinolone acetonide intravitreal implant. The implant has no polyimide shell. It has a zero-order kinetic release rate and is designed to last about six months.
Advertisement
The DAVIO 2 study randomized patients to receive two different doses of EYP-1901 versus aflibercept. Patients were previously treated for nAMD with anti-VEGF medication over the prior six months. Their BCVA ranged from 85 to 35 letters, and their central subfield thickness was less than 350 microns, with low amounts of intraretinal fluid. Patients received either the investigational treatment or aflibercept on the same day and were followed over time.
The criteria for a supplemental injection were similar to the retreatment criteria we see in a lot of durability studies:
Whether or not these criteria are truly relevant to clinical practice — and whether or not we should wait this long to retreat patients — is debatable.
Regardless, by week 32, patients who were randomized to receive one dose of EYP-1901 (either 2 mg or 3 mg) had nearly the same mean change in BCVA from baseline as the patients treated (and often retreated) with aflibercept during the study. There also was no significant difference among patients’ mean change in central subfield thickness from baseline.
In the 12 months prior to joining the study, these patients had received a lot of treatment for nAMD, a mean of 9-10 injections of an anti-VEGF drug. However, those receiving the 2 mg dose of EYP-1901 had about an 89% reduction in the number of injections during the study. Those receiving the 3 mg dose had an 85% reduction in number of injections. They went from a mean of about five injections in the six months before the study to less than one injection in the six months after. About 65% of patients were able to be maintained on just one injection of EYP-1901, either 2 mg or 3 mg, during the entire course of the study to week 32.
Advertisement
The drug had a nice safety profile overall, with no significant adverse events, no significant migration of the implant, and no retinal vasculitis or occlusive disease. Those are things we always watch for in these newer therapies.
Axitinib is a little more potent than vorolanib. It also is being studied in a bioerodible implant that provides targeted release for nine to 12 months.
In the recent OTX-TKI phase 1 trial, patients with nAMD were randomized to receive either axitinib or aflibercept. Patients had been treated with a mean of eight anti-VEGF injections during the 12 months before the trial. They had very thin retinas, with a mean central subfield thickness of 240 microns to 273 microns.
The criteria for retreatment during this trial was similar to DAVIO 2, above. Again, 60% of patients receiving the investigational drug did not need a supplemental injection up to week 48 of the trial. The mean changes in BCVA and central subfield thickness were comparable between the two arms of the trial — all very stable, which is what you would expect in a previously treated population.
The safety was quite good overall. There was one patient with endophthalmitis, but no significant rises in intraocular pressure or other adverse events.
The study of axitinib is continuing in the phase 3 SOL-1 trial.
The tyrosine kinase inhibitors in these studies seem to provide what we want in clinical practice. We want to give one or maybe two injections of an anti-VEGF and then achieve some durability. It is exciting to see many durable therapies coming our way.
Advertisement
However, patient populations are quite heterogeneous. Durable options may not need to be applied broadly because some patients may not need them. In the CATT trial, we had some patients who got a few anti-VEGF injections and never needed another one. It will be important to make sure we use durable therapies only in the right patients.
Dr. Singh is Vice President and Chief Medical Officer of Cleveland Clinic Martin Health in Stuart, Florida, and a professor of ophthalmology at Cleveland Clinic Lerner College of Medicine. This article was based on his presentation at the 2024 Cleveland Clinic Cole Eye Institute Retina Summit.
Advertisement

New insights on effectiveness in patients previously treated with other anti-VEGF drugs

Evidence mounts that these diabetes and obesity drugs may protect eyes, not endanger them

CFH gene triggers the eye disease in white patients but not Black patients
Study explores association between sleep aid and eye disease
Early data show risk is 73% higher in patients with lupus, 40% higher in patients with rheumatoid arthritis

Switching medications may decrease treatment burden and macular fluid
Why retina specialists should get comfortable with this imaging tool

The advanced stage of diabetic retinopathy is among the most challenging for retinal surgeons