Among latest findings: Topical losartan helps reduce corneal scarring after injury
If federal grants are the lifeblood of medical research, the cornea program at Cleveland Clinic’s Cole Eye Institute and Lerner Research Institute is thriving. The current total of nine National Institutes of Health (NIH) research project (R01) and U.S. Department of Defense grants — all investigator initiated — is not only one of the strongest vital signs of any cornea service in the U.S., it’s a testament to the pluck and persistence of Cleveland Clinic physicians and researchers.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Depending on the year, only 10% to 20% of NIH grant requests are approved,” says refractive and corneal surgeon Steven E. Wilson, MD, Director of Corneal Research at the Cole Eye Institute. “Sometimes the hardest part of being a researcher is just getting funded.”
Most research programs cannot be sustained on internal, departmental funding alone, he notes. R01 grants typically provide up to $300,000 per year in direct funds (for salaries of research staff, test drugs, research models and more), along with $150,000 to $200,000 in indirect funds.
“Whether it’s a microbiology study, a biomechanical study or another project, all lab work is very expensive and often can’t be accomplished without federal backing,” says Dr. Wilson. “The reason Cleveland Clinic is on the leading edge of cornea care is because we have staff who not only have a passion for researching new treatments and cures, but also have the drive to compete for and win federal funding.”
Below are highlights of the cornea research currently in progress at the Cole Eye Institute and Lerner Research Institute thanks to R01 and Department of Defense grants.
Funding sources: Two U.S. Department of Defense grants
Principal investigator: Dr. Wilson
Background: For 30 years, Dr. Wilson’s lab has studied the role of growth factors and growth factor receptors in corneal homeostasis and corneal wound healing (aimed at restoring transparency). Damage to the cornea can occur due to infection, surgical complications and chemical burns, among other insults. The U.S. Department of Defense has funded Dr. Wilson’s work as it relates to corneal injuries sustained in war, which are common causes of disability.
Advertisement
Notable findings to date:
Next steps: Research will continue on the use of topical losartan and other topical medications to treat severe corneal injuries, including combining losartan with corticosteroids and other modulators to prevent corneal neovascularization (a cause of corneal opacity), and to regenerate the corneal endothelium and Descemet’s membrane after injury. Dr. Wilson’s second, recently awarded Department of Defense grant will be used to investigate the role of multiple drugs controlling corneal scarring and the growth of abnormal blood vessels in corneas with and without corneal endothelial and Descemet’s membrane injury.
Advertisement
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7b51efc7-2852-4e40-b088-5efce478deea/22-EYE-2826711-CQD_fig-1_Cornea-Service-Grants_805x604_jpg)
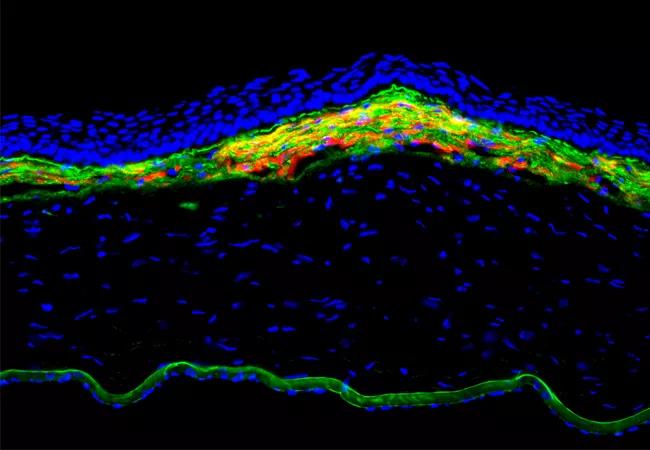
Figure 1. Fibrosis response in the anterior rabbit cornea at 1 month after -9D photorefractive keratectomy. The yellow and orange is alpha-smooth muscle actin-positive myofibroblasts in the anterior stroma of this cornea with fibrosis. Green is basement membrane component nidogen-1 that is produced in stromal cells and is prominently seen in Descemet’s membrane in the far posterior of this cornea. 400X mag.
Funding source: NIH R01 grant
Principal investigator: J. Bradley Randleman, MD, a refractive and corneal surgeon at the Cole Eye Institute
Background: Despite numerous technological advancements in ophthalmology, there is still no direct way to measure corneal biomechanics. Ophthalmologists currently use a variety of indirect measures, such as corneal curvature and thickness, to determine a patient’s eligibility for refractive surgery and risk of developing keratoconus. Brillouin microscopy is a newer technology that uses only light to map corneal stiffness in three dimensions. This information will allow ophthalmologists to be even more precise in patient evaluations, leading to more customized treatments.
Notable findings to date: At the Association for Research in Vision and Ophthalmology (ARVO) 2022 meeting, Dr. Randleman’s team presented findings from two studies:
Advertisement
Next steps: Studies are ongoing and currently enrolling patients.
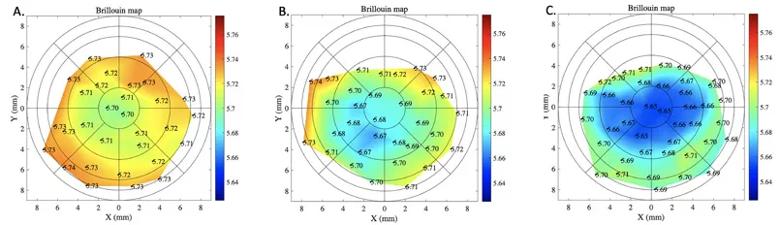
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9d5d9414-6f8d-49d0-b7cd-fd2201170011/22-EYE-2826711-CQD_fig2_Cornea-Service-Grants_805x232_jpg)
Figure 2. Brillouin maps of (A) a normal control cornea, (B) a post- LASIK cornea and (C) a cornea with keratoconus. There are distinct differences centrally in the three corneas, with greatest differences from center to periphery in the keratoconic cornea.
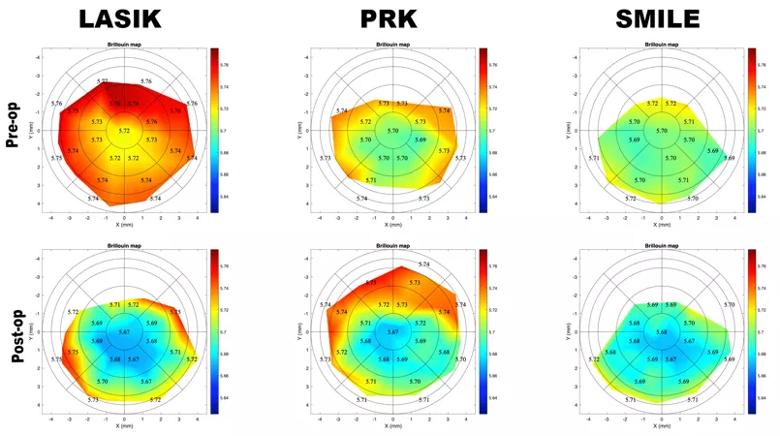
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e1bc4e0f-961c-487c-8904-6441671bc146/22-EYE-2826711-CQD_fig3_Cornea-Service-Grants_805x_450_jpg)
Figure 3. Brillouin maps before (upper) and after (lower) LASIK, PRK and SMILE. There is focal central weakening in each cornea that is most pronounced after LASIK.
Funding source: NIH R01 grant
Principal investigators: Dr. Randleman; William J. Dupps Jr., MD, PhD, a refractive and corneal surgeon at the Cole Eye Institute; and Giuliano Scarcelli, PhD, of the University of Maryland
Background: Corneal cross-linking has been used to halt the clinical progression of keratoconus for more than two decades outside the U.S. It was approved in the U.S. in 2016. To date, there is little direct information about the specific amount of corneal stiffening induced by the standard protocol or any of its variants.
Next steps: This study will begin in mid-2022. The research team’s goals are to:
Advertisement
Hypothesis: The team hypothesizes that different cross-linking techniques will induce different degrees of corneal stiffening. Further, they believe they will be able to predict cross-linking outcomes using Brillouin and finite element modeling once they know the relative impact of different cross-linking procedures. This information ultimately may guide clinical treatments and allow for customized cross-linking approaches, including the ability to safely perform PRK in combination with cross-linking to improve patients’ visual function while strengthening their cornea.
Funding source: NIH R01 grant
Principal investigators: Dr. Dupps; Andrew Rollins, PhD, of Case Western Reserve University; and Rafael Grytz, PhD, of the University of Alabama at Birmingham
Background: This research combines the development of novel tools for optical mapping of an individual’s unique corneal biomechanical properties and creation of patient-specific computer simulations to predict a patient’s surgical outcome or risk of disease development and progression. In addition to addressing important gaps in our understanding of corneal diseases such as keratoconus, this work is expected to produce validated simulation tools that surgeons can use to optimize an individual’s surgical plan prior to performing the actual treatment.
Notable findings to date: This grant begins in mid-2022.With funding from a previous R01 grant, Drs. Dupps and Rollins developed a two-dimensional, displacement-tracking contact OCT technique, optical coherence elastography (OCE), and translated it for human corneal biomechanical measurement. Among other findings, the studies identified novel depth-dependent differences in corneal stromal stiffness in patients with keratoconus that may point to an early disease biomarker.
Next steps: With the new grant, the team will expand OCE into a more comprehensive, three-dimensional elastography method and combine these detailed biomechanical maps in patient-specific finite element models that may help better predict patient outcomes of LASIK, SMILE and PRK, and risk of disease progression in keratoconus. The models will be made available for clinical use through a cloud-based surgical simulation platform that Dr. Dupps and his Cole Eye Institute team are developing (Figure 4).
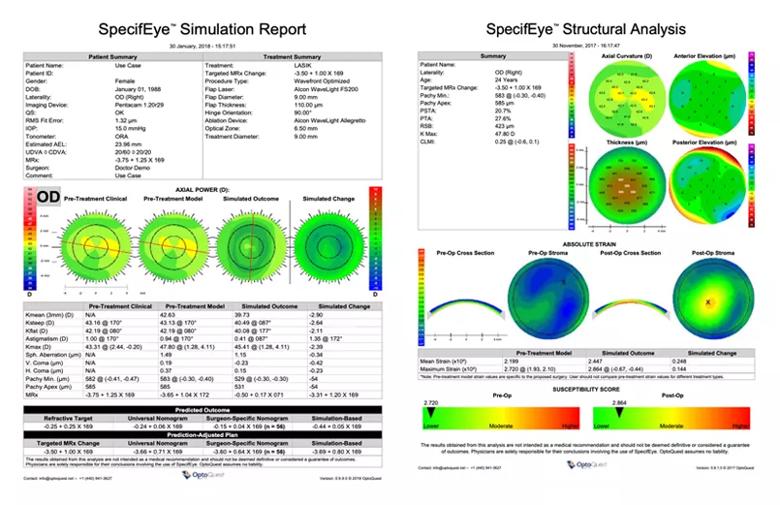
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5f0bbe92-0501-443b-9182-13f3a5df72c2/22-EYE-2826711-CQD_fig4_Cornea-Service-Grants_805x_514_jpg)
Figure 4. Example of finite element model-based patient-specific simulations for predicting a LASIK outcome (left) and the risk of corneal weakening in that eye if LASIK is pursued (right).
Funding source: NIH R01 grant
Principal investigators: Drs. Dupps and Rollins
Background: The purpose and application of this work is similar to the above projects, targeting the goal of spatially resolved corneal biomechanical characterization in live human eyes. This new approach, phase-decorrelation optical coherence tomography (PhD-OCT), involves a noncontact measurement method (like Brillouin microscopy) that can be performed with standard OCT hardware, using only software modifications. The method captures short-term temporal decorrelations in rapidly acquired serial OCT imaging sections that correspond to local tissue stiffness or mobility.
Notable findings to date: A study published in Investigative Ophthalmology & Visual Science describes these findings:
Next steps: In the remaining year of the grant, the team is completing a clinical study with PhD-OCT to measure the effect of cross-linking in patients with keratoconus and to study the effects of refractive surgery procedures that alter corneal biomechanics.
Funding source: NIH R01 grant
Principal investigator: K.P. Connie Tam, PhD, an ophthalmic researcher at the Cole Eye and Lerner Research institutes
Background: Dr. Tam’s ongoing work is focused on better understanding molecular mechanisms governing the cornea’s ability to protect against infection and inflammation. Since 2012, the National Eye Institute (NEI) has funded Dr. Tam’s studies that interrogate the surprising antimicrobial and anti-inflammatory roles of cytokeratin 6a (K6a) protein and its bactericidal peptide fragments (keratin-derived antimicrobial peptides, KAMPs). Structurally, K6a shifts back and forth between its cytosolic subunit form and polymerized filaments of the cytoskeleton in stratified epithelial cells, including those of the cornea (Figure 5).
Notable findings to date:
Next steps: Dr. Tam’s team is currently working to:
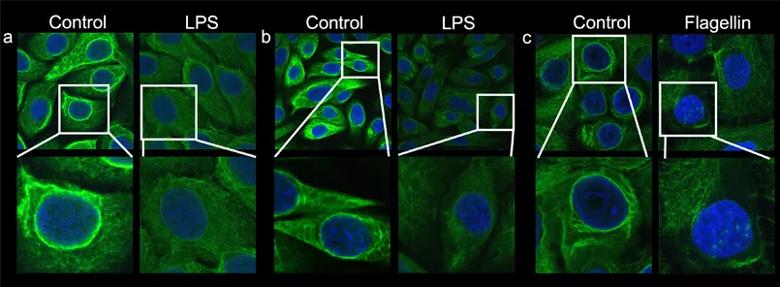
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f3c46231-7206-4f84-ab34-835471913d57/22-EYE-2826711-CQD_fig5_Cornea-Service-Grants_805x_296_jpg)
Figure 5. Multiple normal human epithelial cell lines demonstrate that exposure to inflammatory bacterial molecules prompts depolymerization of the K6a filamentous network, which in turn increases K6a subunits in the cytosolic pool. K6a filaments are labeled green with anti-K6a antibody. The intense green staining of filaments in the cytosol and surrounding the nucleus (blue) becomes diffused and weak after stimulation. (a) corneal epithelial cells, (b) mammary epithelial cells, (c) ecto-cervical epithelial cells.
Funding sources: Two NIH R01 grants
Principal investigators: Dr. Tam and Feng Lin, PhD, a researcher in the Department of Inflammation and Immunity at Lerner Research Institute
Background: In addition to funding Dr. Tam’s work on K6a and KAMPs, the NEI has more recently funded her team’s work to understand how CDCP1, a transmembrane protein and Src kinase activator on corneal epithelial cells, intrinsically regulates the activities of these cells during bacterial keratitis and wound healing.
Notable findings to date: CDCP1 on the corneal epithelial cell surface is activated upon bacterial exposure and wounding to confer protection. The research team has found that protease(s) produced by Pseudomonas aeruginosa cleave the extracellular domain of CDCP1, leading to enhanced production of antimicrobial peptides by corneal epithelial cells, which are important for bacterial clearance. They also have found that CDCP1 cleavage activation after wounding leads to increased production of effector molecules that regulate epithelial cell migration. (Publication pending.)
Advertisement

Motion-tracking Brillouin microscopy pinpoints corneal weakness in the anterior stroma

Registry data highlight visual gains in patients with legal blindness

Prescribing eye drops is complicated by unknown risk of fetotoxicity and lack of clinical evidence

A look at emerging technology shaping retina surgery

A primer on MIGS methods and devices

7 keys to success for comprehensive ophthalmologists

Study is first to show reduction in autoimmune disease with the common diabetes and obesity drugs

Treatment options range from tetracycline injections to fat repositioning and cheek lift