NIH-funded study explores novel MRI technique to stage cystic kidney disease
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic kidney disorder, occurring in approximately 1 in 1,000 individuals. Affected patients may experience significant morbidities, including progressive chronic kidney disease (CKD). ADPKD accounts for 10% of end-stage kidney disease (ESKD) in those < 65 years of age. Unfortunately, standard clinical measures of kidney function, such as estimated glomerular filtration rate (eGFR), typically do not show changes until cystic kidney disease is very advanced and substantial damage has occurred over many years, highlighting the need for additional biomarkers to identify patients at high risk for progression and monitor the effect of novel therapies.
The Consortium for Radiological Imaging Studies In Polycystic Kidney Disease (CRISP) and others established height-corrected total kidney volume (htTKV), obtained from magnetic resonance imaging (MRI), as an effective outcome measure in clinical trials of adults with ADPKD.1 htTKV, in addition to eGFR, was utilized in clinical trials of tolvaptan, a novel vasopressin receptor antagonist, resulting in the first-ever FDA approval of a medication to treat ADPKD in adults. htTKV is also used clinically in adult ADPKD to identify patients at high risk for disease progression using the Mayo Imaging Classification (MIC) schema.2
Development of effective therapies for children with ADPKD has the potential to limit kidney disease progression at an earlier time point, resulting in improved preservation of kidney function and, ultimately, a longer life expectancy. Unfortunately, there is no established method to measure kidney disease progression in these children, as htTKV has not been validated in ADPKD patients < 15 years of age. Assessment of progression based on kidney volume is complicated by the normal growth of kidneys during childhood. Thus, for an individual ADPKD child, it may be difficult to determine if kidney growth is appropriate for age or represents more rapid growth associated with higher risk of disease progression as seen in adults. Because of this significant limitation, FDA-approved treatment and multiple ongoing clinical trials of novel agents are unavailable for children with ADPKD.
Advertisement
Our multidisciplinary group at Cleveland Clinic Children’s and Case Western Reserve University (CWRU), including my longstanding collaborator, Dr. Chris Flask (MRI research), is currently conducting an NIH-funded prospective observational study to develop quantitative MRI biomarkers for the rarer, autosomal recessive form of PKD (ARPKD) (R01-DK114425). As part of this research, we have applied a novel MRI technique developed at CWRU, called MR Fingerprinting (MRF)4 to the study of ARPKD. Our preclinical and clinical studies show that T1 and T2 mapping, obtained by MRF, can reproducibly stage disease across a spectrum of cystic kidney disease severity and potentially measure response to therapy.5-7
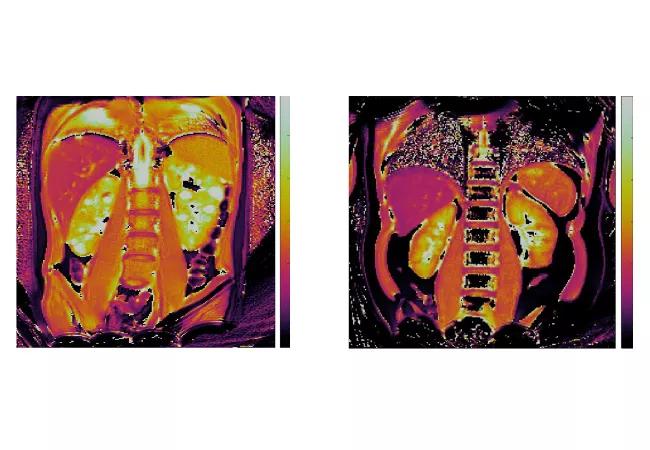
Building on this work and, in collaboration with Dr. Arlene Chapman, an established adult nephrology ADPKD investigator at University of Chicago, we have begun an NIH-funded pilot study in children and young adults with ADPKD (R56-DK133427). For these initial studies, we are developing MRF-based methods, including novel MRF-radiomics, to characterize cystic and non-cystic kidney parenchyma. Representative MRF-derived T1 and T2 maps from an ADPKD child and a healthy volunteer are illustrated in the figure below.
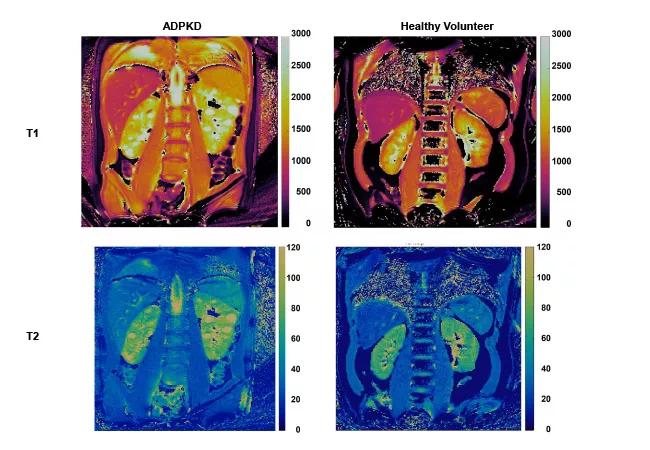
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/078b3ccd-90a8-40e7-a300-0cdc6dc33bdb/23-CHP-3820958-CQD-Dell-Perspectives-Article_jpg)
Figure. Magnetic resonance fingerprinting (MRF) of pediatric ADPKD kidney disease. MRF-based T1 maps (top panels) and T2 maps (bottom panels) for an ADPKD child (left panels) and a healthy volunteer (right panels). Note the increased kidney T1 and T2 values that correspond to discrete kidney cysts in the ADPKD patient.
Advertisement
The long-term goal of this project is to improve clinical care and access to novel therapies for children with ADPKD by establishing an MRI-based risk classification system for patients < 15 years of age, which could be used to identify those at high risk for progression for inclusion in clinical trials and monitor response to therapy. Because MRF is inherently resistant to motion, MRF may allow even young children to be included in clinical trials that utilize MRI, without the need for sedation. In addition, since ADPKD is an inherited disorder, we also hope to understand better the extent to which a parent’s disease severity (MIC score) can be used to predict the child’s clinical course, which may also identify “high-risk” patients and allow for earlier interventions, when available.
About the author: Dr. Katherine Dell is the Director of Clinical and Translational Research at Cleveland Clinic Children’s and a Professor of Pediatrics at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University. She is a member of the Center for Pediatric Nephrology and Hypertension and is an established investigator and internationally recognized expert in pediatric cystic kidney diseases.
References
Advertisement
Advertisement

One pediatric urologist’s quest to improve the status quo

First single-port renal vein transposition reduces recovery time and improves outcomes
Fully-automated process uses preop CT, baseline GFR to estimate post-nephrectomy renal function
Belzutifan superior to everolimus in phase 3 clinical trial
Management of high-risk RMSK in the pre-and current eras of neoadjuvant therapy
AI-generated model bests predictive abilities of human experts
Study highlights benefits of nephrologist-led urine sediment analysis
Using sequencing data to identify novel factors linked to kidney disease with unknown origin