New international study paves the way for imminent update of the ILAE classification
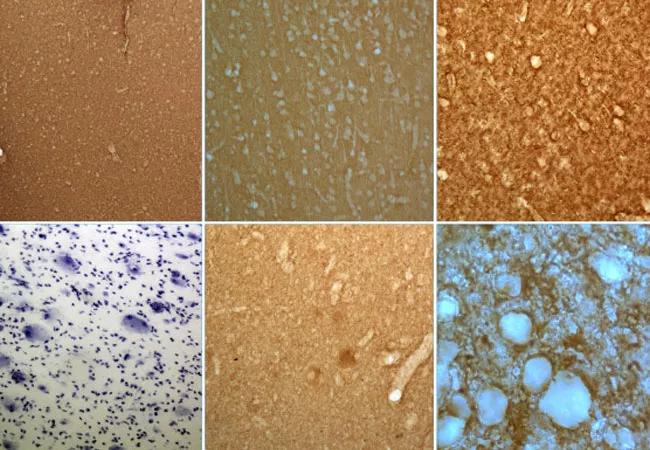
Clinicians awaiting the forthcoming update to the International League Against Epilepsy (ILAE) classification of focal cortical dysplasia (FCD) can expect a panel of suggested immunohistochemical stainings for improved histopathological workup and the introduction of a proposed gene panel to improve diagnostic precision.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
So suggests the recent publication of an iterative histopathological and genetic agreement trial by members of the ILAE Task Force on Focal Cortical Dysplasia (Epilepsia. 2021 May 5). “We undertook this study as preparatory scientific work to lay the foundation for recommendations in the upcoming update to the FCD classification,” says Cleveland Clinic Charles Shor Epilepsy Center Director Imad Najm, MD, who serves as chair of the ILAE’s FCD Classification Task Force. He is the study’s senior author and will be first author of the upcoming FCD classification update.
Many cases of difficult-to-treat epilepsy are attributable to malformations of cortical development — i.e., abnormalities in the alignment of neurons and cells in the cortex that occur during embryonic development. FCD represents nearly three-quarters of all cases of these malformations in patients who undergo evaluation for epilepsy surgery.
Publication of the ILAE’s initial classification scheme for FCD in 2011 (Epilepsia. 2011;52:158-174) was a watershed development in the diagnosis and management of FCD. “Since its publication, more than 1,000 papers have used the ILAE classification of FCD for reporting on the pathology, clinical manifestations, electrophysiology, imaging, genetics or surgical outcomes in patients with difficult-to-treat epilepsy,” says Dr. Najm.
Despite the classification’s widespread use, its limitations have become clearer as more than a decade has passed since its development. These include challenges in inter- and intraobserver agreement on histopathological diagnosis (particularly for FCD type 1), and scant integration of genetic and molecular findings into diagnostic considerations, which are currently based on microscopic review of surgical brain tissue using the 2011 classification protocol.
Advertisement
These limitations prompted recent efforts to produce an updated FCD classification scheme, which is expected to be published by the end of 2021. The recent study in Epilepsia was conducted to inform those efforts by identifying specific areas of diagnostic challenges using an iterative histopathological agreement methodology supplemented with genetic testing.
Well-characterized brain tissue samples were obtained from 22 consecutive patients who underwent epilepsy surgery at Cleveland Clinic for difficult-to-treat epilepsy with a disease etiology within the spectrum of FCD.
These samples underwent a series of four web-based pathology agreement trials (using digitized images) completed by 20 neuropathologists from 15 nations. The final trial was supplemented by genetic tests conducted by five independent genetic laboratories around the world; these tests involved screening or validation sequencing of FCD-relevant genes in paired brain and blood samples from the same 22 epilepsy surgery patients.
Round 1 of the trial involved histopathological assessment based exclusively on hematoxylin and eosin (H&E) staining, and it yielded low interrater agreement except for cases of FCD type 2.
Round 2 included a panel of additional immunostainings, and Round 3 introduced the Delphi consensus method — i.e., informed decision-making based on anonymous disclosure of all previous results — as well as a summary of patients’ clinical information, including preoperative MRI and electrophysiological findings. These rounds produced modest incremental gains in interrater agreement, primarily for FCD types 2 and 3.
Advertisement
Round 4 introduced results from the genetic tests and yielded good interrater agreement, with nearly 100% concordance for FCD type 2 and notable gains in concordance for other subtypes.
The researchers made several overarching observations:
“This study showed that interobserver agreement on FCD classification increased when immunohistochemistry was available to neuropathologists and when results of genetic tests were available,” says Dr. Najm. “In view of this, our task force will be including in the ILAE classification update a panel of immunohistochemical stainings for better definition of FCD subtypes and a gene panel to promote comprehensive and integrative genotype-phenotype diagnosis.”
In their study report, the authors also propose incorporating a “no FCD” or “normal histology” category into the classification framework to allow for cases where no FCD diagnosis can be made with confidence using current histologic and genetic capabilities, despite MRI findings suggestive of FCD.
Advertisement
Dr. Najm explains that introducing a normal-histology category would reduce the current tendency for FCD type 1 to be used as a sort of wastebasket into which inconclusive cases are classified. “Classifying these cases as FCD type 1 removes the incentive for additional research to further characterize them,” he says.
He adds that the addition of a normal-histology category is consistent with a new tiered approach to the FCD classification scheme that the task force plans for the upcoming update. The tiered approach is modeled on the multilayered diagnostic scheme recently adopted for the World Health Organization Classification of Tumors of the Central Nervous System, which has helped move brain tumor care toward a precision medicine approach.
Dr. Najm says it’s helpful to think of the tiered approach as a table with multiple fields — histology, genetics, MRI findings, other clinical information — that are filled in one at a time as information becomes available. Until the findings point to a definitive classification, a case can be labeled in comprehensive descriptive terms, such as “FCD type 2A left frontal lobe with positive MRI.” He says this approach reduces the tendency to overdiagnose and allows for flexibility and nuance as additional information becomes available or as new markers or diagnostic capabilities emerge.
“The classification from 2011 was largely about histopathological findings,” Dr. Najm notes. “The upcoming classification update will put histopathology in the context of everything else, including genetic, molecular and imaging findings. This is fundamental because it represents the first time international experts in neuropathology, epilepsy, epilepsy surgery, imaging, molecular biology and genetics have come together to work on the FCD classification. We believe this comprehensive approach is going to lead to a more accurate and clinically meaningful subclassification of these dysplasias, because some of them that may look the same under the microscope might not look the same when genetic testing is brought to bear. And the new insights can have big implications for patient counseling and for treatment and outcomes, leading us to one treatment over another.”
Advertisement
Dr. Najm says this impact on management will be the ultimate payoff for epilepsy patients and their treating neurologists in the community. “The tiered approach we are advocating has a clear role for community neurologists,” he adds, “as it starts with them when they obtain an MRI that shows characteristics of one or another FCD subtype. Community neurologists will be filling the first fields in the table under the tiered approach, which will help them refer patients with suspected FCD for optimal subsequent management. This is an important step toward the development of a precision medicine approach to FCD.”
Advertisement

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

An expert’s take on evolving challenges, treatments and responsibilities through early adulthood

Comorbidities and medical complexity underlie far more deaths than SUDEP does

Novel Cleveland Clinic project is fueled by a $1 million NIH grant

Tool helps patients understand when to ask for help