Timing of adjuvant immunotherapy unclear for maximum overall survival
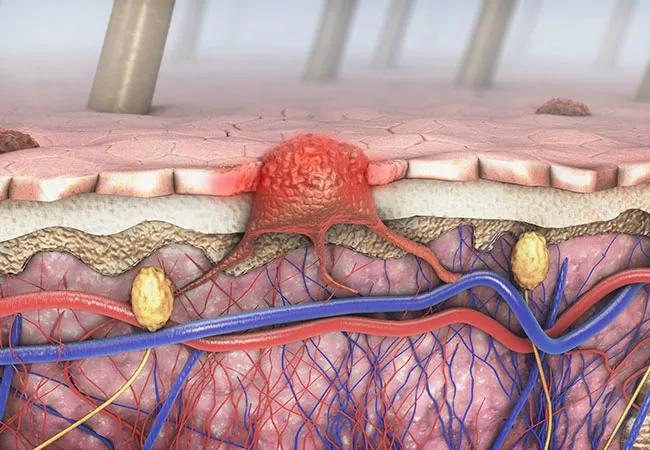
Research has shown adjuvant immunotherapies can quadruple the survival rate for patients with stage IV melanoma, so when a new Phase III study revealed no overall survival benefit for patients with stage II fully-resected disease, researchers were confounded.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The SWOG-1404 trial is one among several that aimed to better understand the role that adjuvant immunotherapies play in treating surgically-resected melanoma. After comparing pembrolizumab with high-dose IFNa-2b or ipilimumab for 1,301 patients with stage II/III fully-resected disease, the researchers found a statistically significant improvement in recurrence-free survival but not one in overall survival.
The study also assessed the use of pembrolizumab in the adjuvant setting against high-dose interferon or the investigator’s choice of ipilimumab for patients with resected stage III/IV melanoma. The 3.5-year study follow-up demonstrated that the patients receiving pembrolizumab over a 12-month period achieved significantly higher relapse-free survival.
Despite lacking a demonstrable overall survival benefit, the study did reveal clues that may help researchers in understanding how and when to employ immunotherapies for patients in the adjuvant setting.
New data is emerging that suggests the benefit of immunotherapy may be higher if it’s started prior to surgery. “If you consider the fact that immunotherapy uses the immune system to recognize and attack a tumor, it makes sense that employing it prior to resection may be a better alternative,” says Pauline Funchain, MD, SWOG-1404 study co-author and an oncologist/cancer genomics expert at Cleveland Clinic. “More data is needed to know for sure whether neoadjuvant or adjuvant treatment will improve overall survival.”
Another key question for clinicians is whether patients are better off being prescribed adjuvant immunotherapy at stage III to lower the risk of recurrence or whether the potential for adverse events should preclude them from doing so. The answer depends on each situation.
Advertisement
Although they are rare, it is possible for permanent side effects such as hypothyroidism or adrenal insufficiency to occur in patients receiving immunotherapies. “The answer to whether the benefit of immunotherapy outweighs the risk depends on each patient sitting in front of you,” says Dr. Funchain. “For a patient who doesn’t like seeing doctors or who might have a side effect like arthritis that changes their quality of life, they may not want to take that risk, but other patients want the ability to sleep at night knowing they’ve done everything possible to extend their life.”
This conversation is also relevant for patients with stage II disease, which has a smaller risk of disease recurrence. “When you look at the benefit of immunotherapy for recurrence-free survival versus the chance of contracting a permanent side effect such as hypothyroidism, they are almost equal,” notes Dr. Funchain.
The treatment landscape for melanoma has improved considerably in recent years, necessitating conversations with patients about the best option for their individual situation. “Just 15-20 years ago, there were few options for patients with melanoma,” says Dr. Funchain. “Today, one patient might have three different viable options, so as clinicians we can’t just prescribe the ‘right’ thing to do because the right thing will vary for each patient. It’s becoming more and more important in oncology as more therapies become available that we have those conversations with our patients and involve them in the decision-making process so they understand each of their choices.”
Advertisement
Advertisement

Neoadjuvant immunotherapy improves outcomes

Largest study of its kind identifies three treatment exposures that contribute to risk
Family history may eclipse sun exposure in some cases
Immunotherapy is preferred initial treatment for majority of patients

Melanoma may be more heritable than we think

Why Cleveland Clinic is launching its cardioimmunology center

Collaborative patient care, advanced imaging techniques support safer immunotherapy management

Pembrolizumab does not improve outcomes, but immunotherapy may still offer benefit