Radiomics analysis suggests underlying subvisual features may encode information related to tolerance of interval extension in retinal vascular disease
Is it possible to use imaging features to enhance the current “treat-and-extend” approach to determining proper dosing of anti-VEGF medication for patients with retinal vascular disease? Cleveland Clinic and Case Western Reserve University researchers may have found a new opportunity to bridge from imaging to personalized therapy: two radiomics-based ultra-widefield angiographic biomarkers that can differentiate between eyes that need more frequent dosing and those that don’t.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Their radiomics study, recently published in the British Journal of Ophthalmology, indicated that leakage patterns and tortuosity in retinal blood vessels on ultra-widefield angiography are associated with tolerance of interval extension for anti-VEGF therapy in eyes with diabetic macular edema or retinal vein occlusion.
“Few studies have looked at quantifying these features in this way, in part due to capabilities in image analysis technology that haven’t become available until recently,” says Justis P. Ehlers, MD,Director of The Tony and Leona Campane Center for Excellence in Image-Guided Surgery and Advanced Imaging Research at Cleveland Clinic’s Cole Eye Institute.
This study was generated from a Cleveland Clinic study, PERMEATE, analyzing the ultra-widefield angiography feature dynamics in eyes undergoing treatment with anti-VEGF therapy for diabetic macular edema or retinal venous occlusive disease. The leakage and retinal vascular segmentation was enabled with image analysis software developed by Cleveland Clinic. Radiomics features were extracted and analyzed under the expertise of Anant Madabhushi, PhD, Director of the Center for Computational Imaging and Personalized Diagnostics at Case Western Reserve University. The collaboration between Drs. Ehlers and Madabhushi led to the recent findings.
“We know that multiple leakage patterns exist in retinal vascular disease, but computational analysis of these patterns has been limited,” says Dr. Ehlers. “Utilizing a leakage identification tool, the leakage foci were identified and outlined. This allowed for computational radiomics extraction to evaluate the distribution of those leakage nodes, a novel approach to leakage morphology assessment and pattern characterization.”
Advertisement
The study then assessed the biologic relevance of the leakage distribution pattern. Baseline images showed differences between eyes that tolerated less frequent anti-VEGF treatment and those that didn’t.
“We were able to use pre-treatment images to differentiate treatment response eight months later,” says Dr. Ehlers. “That was one of the more exciting findings. If we could be more forward-thinking in predicting treatment durability, it would be a huge advance for personalized therapy and potentially for new therapeutic development through early identification of eyes that may not respond optimally to a given therapy.”
Anti-VEGF therapy is well-established as the gold standard for retinal vascular disease, particularly macular edema due to diabetic eye disease or retinal vein occlusion. Optical coherence tomography (OCT) is used regularly to inform treatment decisions, including determining whether a patient needs to be treated at all or if treatment needs to be changed. While OCT provides detailed information on the retinal anatomy (and its changes with treatment over time), it does not provide information about retinal vasculature status and leakage dynamics.
Ultra-widefield angiography now enables near panretinal visualization of vascular features, including ischemia and leakage. One key challenge in angiography assessment is the availability of objective quantification of features.
“One major focus of our research has been to develop a platform to automatically quantify features on ultra-widefield angiography, such as leakage, ischemia and micro-aneurysms,” says Dr. Ehlers. “Our primary goal was to devise a tool that could provide a quantitative assessment of disease burden and treatment response.”
Advertisement
The PERMEATE study, published in Ophthalmology Retina in early 2020, was designed to use this tool to evaluate the longitudinal change in quantitative ultra-widefield angiographic features. Dr. Ehlers and his team studied 31 eyes, all with macular edema from either diabetic eye disease or retinal vein occlusion. All had an intravitreal aflibercept injection every four weeks for six months. Then the treatment interval was extended to every eight weeks for six more months. During the study, patients had ultra-widefield angiography performed at multiple key time points.
“In this original analysis, we used the software system to quantify areas of leakage and ischemia, tracking how they responded to treatment,” says Dr. Ehlers.
The current study was a continuation of this work. Researchers classified the eyes as either “rebounders” (those whose visual acuity decreased after the first eight-week treatment interval) or “non-rebounders” (those whose visual acuity improved or stayed the same after the first eight-week treatment interval).
Using the segmented masks of interest from the ultra-widefield angiography images, the team partnered with Dr. Madabhushi to explore features — even subvisual ones — that could have biologic relevance.
Dr. Madabhushi’s radiomics quantification team had previously studied the importance of vascular tortuosity in other conditions, such as tumors. In this analysis, quantitative retinal vascular tortuosity measurement was selected based on the known association of high levels of VEGF with increased vascular tortuosity.
Advertisement
“Our team identified that baseline tortuosity was more complex in eyes that worsened with extended interval dosing,” says Dr. Ehlers. “This wasn’t something readily visible on the images and required computational analysis to identify.”
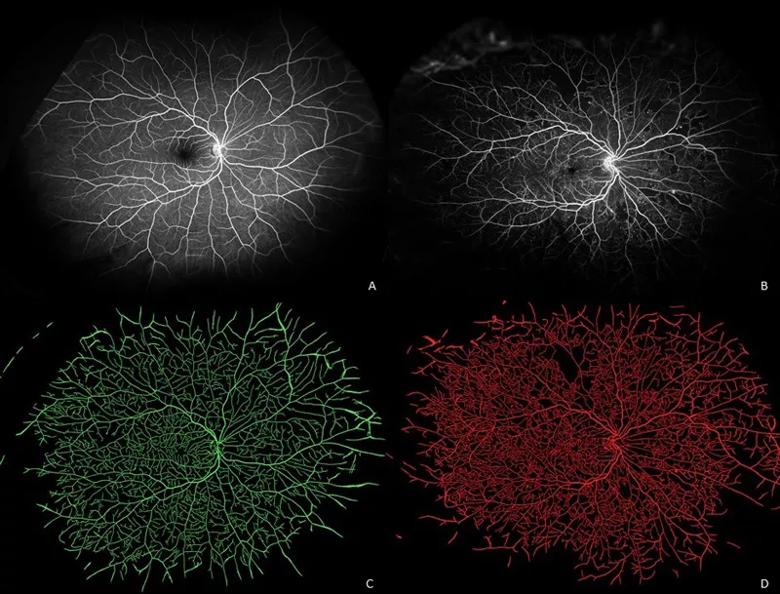
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e1ccf027-7ef3-4a2a-8686-a908ca3ef682/20-EYE-1961371-CQD-AI-radiomics-in-diabetic-eye-disease-Inset-1_jpg)
Ultra-widefield fluorescein angiograms of an eye with minimal retinal pathology (A) and an eye with early proliferative diabetic retinopathy (B) and their corresponding vessel segmentations (C, D). Eye with minimal pathology demonstrates generally uniform vascular distribution (green), while eye with advanced diabetic eye disease demonstrates both multiple areas of capillary dropout and localized increased density/tortuosity (red).
The team also explored leakage patterns and morphology characteristics in the context of treatment response.
“In previous studies, we identified quantitative leakage assessment to be associated with numerous disease features, including disease severity and risk of disease-related complications,” says Dr. Ehlers. “Leakage is a key marker of disease activity and associated with inflammation and VEGF levels.”
In this radiomics analysis, the team found that baseline leakage node distribution/morphology was able to be distinguished in eyes that tolerated treatment interval extension compared to eyes that worsened following treatment interval extension.
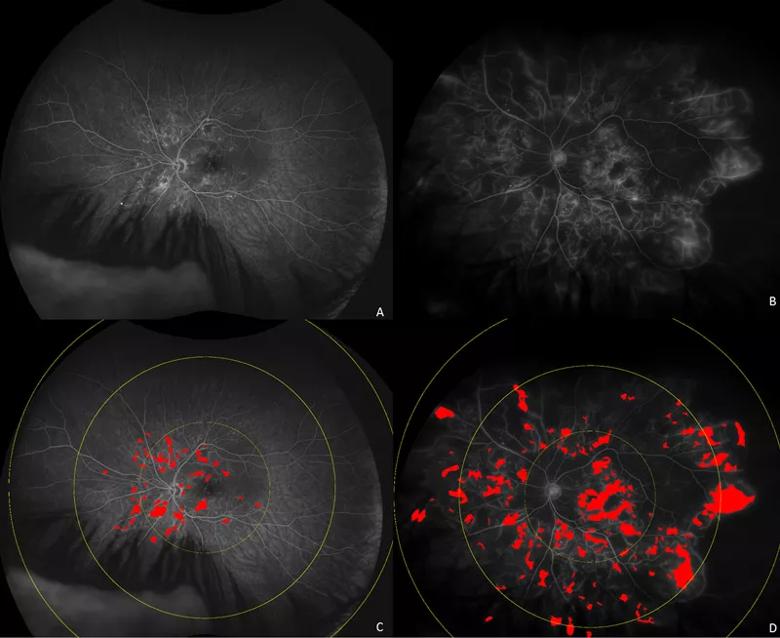
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9d7dcc1b-36a7-4243-b925-a8d6f9973b53/20-EYE-1961371-CQD-AI-radiomics-in-diabetic-eye-disease-Inset-2_jpg)
Ultra-widefield fluorescein angiograms (A, B) of eyes with diabetic macular edema demonstrating different distribution of leakage patterns. Automated leakage segmentation is shown in red overlay for eye with posterior pole dominant leakage (C) and diffuse perivascular leakage sparing peripapillary area (D).
Advertisement
This study is one of the first to exhibit the power of radiomics in ophthalmology, says Dr. Ehlers.
“We couldn’t have done this 10 years ago,” he says. “Today, machine learning is enhancing our accuracy as well as efficiency of our segmentation platform and analysis processes. Adding layers of handcrafted radiomics feature assessment provides unique opportunities to interrogate images for phenotypic features that are associated with prognosis and treatment response. Dr. Madabhushi and his team have been leaders in the field of radiomics and computational medicine. It has been exciting to build a wonderful collaboration in retinal disease assessment with Dr. Madabhushi.”
Because this study was relatively small (N = 27) and combined two distinct retinal vein diseases, larger targeted follow-up studies are needed to validate the findings.
While widespread application of this technology is not currently feasible, this work suggests that diagnostic imaging and higher order characterization could provide valuable data regarding prognosis and likely therapeutic response, says Dr. Ehlers.
“Some patients need regular anti-VEGF injections, possibly for the rest of their lives. Others need only a few injections. And the vast majority of patients are somewhere in the middle,” he says. “But who is who? Currently it takes a lot of trial and error to figure that out in our treatment regimens. I inform patients that we need significant time (often up to a year) to determine their ideal dosing regimen using our traditional treat-and-extend approach. In the meantime, we’re potentially overtreating some patients while undertreating others who are at risk of vision loss.”
Imaging biomarkers could be a game-changer, he says, leading to personalized treatment regimens and even alternative therapeutics.
Advertisement

Motion-tracking Brillouin microscopy pinpoints corneal weakness in the anterior stroma

Registry data highlight visual gains in patients with legal blindness

Prescribing eye drops is complicated by unknown risk of fetotoxicity and lack of clinical evidence

A look at emerging technology shaping retina surgery

A primer on MIGS methods and devices

7 keys to success for comprehensive ophthalmologists

Study is first to show reduction in autoimmune disease with the common diabetes and obesity drugs

Treatment options range from tetracycline injections to fat repositioning and cheek lift