Findings establish overweight/obesity as a modifiable risk factor for cardiovascular disease
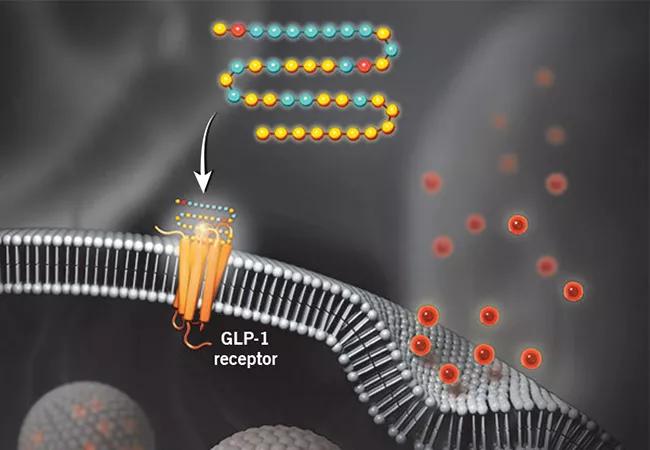
For the first time, a pharmacotherapy for overweight and obesity has been shown to reduce cardiovascular events in patients with established cardiovascular disease (CVD) in the absence of type 2 diabetes.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The finding, achieved with weekly injections of the GLP-1 receptor agonist semaglutide (Wegovy®, Ozempic®) in the multicenter SELECT trial, establishes overweight/obesity as a modifiable risk factor for CVD, according to lead investigator A. Michael Lincoff, MD.
“It is well established that overweight and obesity increase a person’s risk of cardiovascular events, but the concept of treating obesity to reduce cardiovascular complications has been hampered by lack of evidence that lifestyle or pharmacologic interventions for overweight or obesity improve cardiovascular outcomes,” says Dr. Lincoff. “This marks the first pharmacologic intervention for overweight or obesity that’s been shown in a rigorous fashion to reduce the risk of cardiovascular events.”
Dr. Lincoff presented the study findings in a late-breaking science session at the American Heart Association’s Scientific Sessions 2023; they were simultaneously published in the New England Journal of Medicine. The results showed a 20% reduction in the risk of a composite of cardiovascular death, nonfatal myocardial infarction (MI) or nonfatal stroke relative to placebo over approximately 40 months of follow-up.
Semaglutide is approved by the FDA for treatment of type 2 diabetes and for chronic weight management in adults with obesity or overweight. It has been demonstrated to reduce body weight by a mean of 15% among patients with overweight or obesity without diabetes. Moreover, semaglutide and other GLP-1 receptor agonists have been shown to reduce the risk of adverse cardiovascular events in patients with diabetes, but they had not been tested for this purpose in patients with no history of diabetes. The GLP-1 receptor agonists’ cardiovascular benefits are believed to stem from their effects in reducing dysfunctional body fat cells, improving glycemic control, reducing blood pressure and inflammation, and perhaps improving vascular endothelial function and plaque stability.
Advertisement
SELECT was designed to determine whether semaglutide can exert protective cardiovascular effects in patients with overweight or obesity in the absence of diabetes. It enrolled 17,604 adults aged 45 or older with a body mass index (BMI) of 27 kg/m2 or greater and preexisting CVD (prior MI, prior stroke or peripheral artery disease) without diabetes. The cohort’s mean BMI was 33.3 kg/m2, and 71.5% of patients met the criterion for obesity (≥30). The mean glycated hemoglobin level was 5.8%. Mean patient age was 61.6 years, and the cohort was predominantly male (72%) and white (84%).
Patients were randomized 1:1 to receive subcutaneous semaglutide 2.4 mg once weekly or placebo as an adjunct to standard-of-care therapy for CVD, such as cholesterol-modifying medications, antiplatelet therapies and antihypertensive agents. The primary outcome measure was a composite of death from cardiovascular causes, nonfatal MI or nonfatal stroke in a time-to-first-event analysis.
The mean duration of exposure to semaglutide or placebo was 34.2 ± 13.7 months; the mean length of follow-up was 39.8 ± 9.4 months.
The composite primary endpoint occurred in 6.5% of patients in the semaglutide group versus 8.0% of patients in the placebo group (hazard ratio [HR] = 0.80; 95% CI, 0.72 to 0.90; P < 0.001). Consistent trends were observed for each component of the composite endpoint and for all-cause death.
There were no unexpected safety issues with semaglutide. More patients discontinued semaglutide than placebo (16.6% vs. 8.2%; P < 0.001), due primarily to gastrointestinal symptoms (nausea, vomiting and diarrhea) that previously have been reported with GLP-1 receptor agonists. There was a slightly higher rate of gallbladder disorders in the semaglutide group (2.8% vs. 2.3%), which has also been observed in other studies of GLP-1 receptor agonists. Notably, semaglutide was not associated with higher risks for severe gastrointestinal disorders, pancreatitis, psychiatric disorders or kidney injury.
Advertisement
Mean body weight loss in the semaglutide group at 104 weeks was 9.39% (versus 0.88% in the placebo group).
“This study demonstrates the effectiveness of a new pathway to reduce the excess risk associated with obesity of important and potentially deadly cardiovascular complications,” Dr. Lincoff says.
In addition to the primary results noted above, he identified several other noteworthy observations from the study:
The researchers note that while the mechanisms behind semaglutide’s cardioprotection are uncertain, the consistent effects across various cardiometabolic risk factors suggest that multiple interconnected pathways may be involved.
Although this large, rigorous trial can provide support for a potential new indication for semaglutide for secondary prevention of CVD, whether and how quickly payers may reimburse for such an indication is uncertain. Discussion at the American Heart Association session underscored the stakes of such decisions, as it was noted that an estimated 6.6 million American adults meet the inclusion criteria for the SELECT trial.
Advertisement
If and when semaglutide and other GLP-1 receptor agonists are widely used for cardiovascular prevention in patients with overweight and obesity without diabetes, cardiologists will need to gain familiarity with their use. “These drugs require dose titration, particularly to reduce adverse effects like nausea and diarrhea that are common soon after starting therapy,” Dr. Lincoff notes. “Cardiologists will need to counsel patients through that and acclimate them to giving these drugs by subcutaneous injection.”
Meanwhile, he adds, future research is needed to address whether semaglutide exerts the same protective effects for primary prevention of cardiovascular disease in high-risk individuals with overweight or obesity.
“We have been waiting for this trial,” says Leslie Cho, MD, Co-Section Head of Preventive Cardiology at Cleveland Clinic. “The question of whether weight loss on top of great medical therapy can lower events has been answered, and the effect is impressive. Yet the question of cost is an important one. These drugs cost $1,400 a month and most patients need to take them for life to achieve sustained weight reduction. We look forward to additional cardiovascular outcome trials in patients with obesity.”
The trial was sponsored by Novo Nordisk, the company that markets semaglutide. Dr. Lincoff has received consulting fees from Novo Nordisk.
Advertisement
Advertisement

Cleveland Clinic pioneers a new paradigm in cardiovascular care delivery

Site visits offer firsthand lessons in clinical and operational excellence in cardiovascular care

Cancer drug helps treat decades-long symptoms in patient with complicated lymphatic issue

Findings support emphasis on markers of frailty related to, but not dependent on, age
Observational study supports adding CAC score to traditional risk factors for precision medicine approach
Visual snapshots of how we manage challenging cases
Inflammation found more predictive of events than LDL-C in pooled analysis of RCTs
How our HVTI Advisory Services team facilitated swift improvements for an affiliated health organization