Authors summarize the recent evidence and offer two clinical scenarios
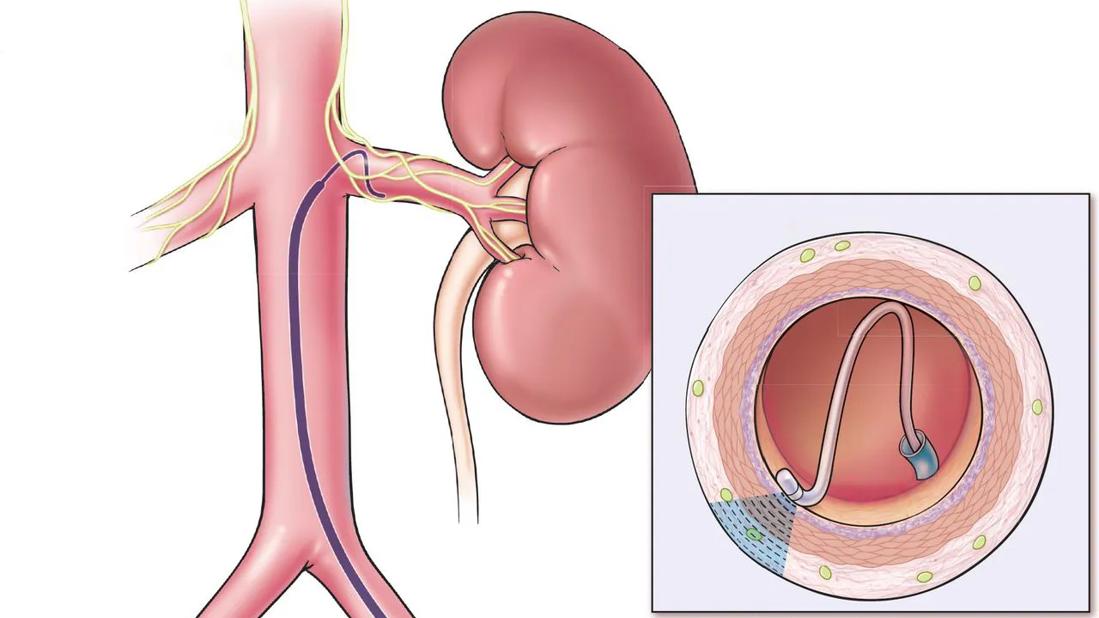
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/dd0e539d-2a56-4d50-b629-e27420996ead/kidney-ablation-catheter-feature)
Illustration depicts renal denervation
By Leen Al-Yacoub, MD; Elias Bassil, MD; George Thomas, MD; Luke Laffin, MD; Aravinda Nanjundappa, MBBS, MD; Khaled Ziada, MD; and Ali Mehdi, MD, MEd
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Editor’s note: This article originally appeared in the Cleveland Clinic Journal of Medicine (2024;91[9]:539-543)
Maybe. Select patients should be referred after informed and shared decision-making. Patients with treatment-resistant hypertension or intolerance to further medication adjustments may be suitable candidates for renal denervation, as it demonstrates a blood pressure (BP)-lowering effect of 5 to 7 mm Hg, comparable to the effect of adding another antihypertensive agent (Figure 1).1–5 Two renal denervation systems — ultrasound and radiofrequency based — are currently approved by the US Food and Drug Administration.6,7 The European Society of Hypertension updated guidelines8 state that renal denervation is a consideration for true treatment-resistant hypertension and for patients with drug intolerances and an estimated glomerular filtration rate (eGFR) greater than 40 mL/minute/1.73 m2.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4db45c07-83d4-4539-a498-bebda4941492/kidney-ablation-catheter-inset)
Figure 1. Renal denervation
It is important to discuss with patients that renal denervation serves as an additional option in the antihypertensive arsenal, but it does not cure hypertension. Discussions to set realistic expectations surrounding BP reduction should be had with the patient specifically regarding the need to continue diet and lifestyle modifications and most of their current pharmacotherapy. The importance of shared decision-making is highlighted in these guidelines.
Of note, apparent treatment-resistant hypertension is defined as uncontrolled BP (daytime mean systolic BP ≥ 135 mm Hg) while taking at least 3 optimally dosed (or maximally tolerated) antihypertensive agents, including a diuretic, or controlled hypertension requiring 4 or more medications.9 When a patient presents with apparent treatment-resistant hypertension, it is critical to rule out pseudoresistance, as these patients may not require any further intervention. White coat hypertension or white coat effect (higher BP in office than at home) is ruled out by evaluating out-of-office BP control. Other contributors to pseudoresistance include improper BP measurement, suboptimal pharmacotherapy, and medication nonadherence.
Advertisement
In the SYMPATHY (Renal Sympathetic Denervation as a New Treatment for Therapy Resistant Hypertension) trial,10 investigators assessed renal denervation vs usual care and medication adherence. Physicians and participants were unaware of the adherence assessment, circumventing the Hawthorne effect. Eighty percent of patients were not adherent to the prescribed regimen, with fewer medications detected than prescribed; on average, two medications were detected in blood or urine samples as opposed to the four prescribed.10 Ruzicka et al9 assessed treatment adherence via directly observed therapy in patients with apparent treatment-resistant hypertension. Resistant hypertension resolved in 30% of patients.
Medication nonadherence can be quite challenging, particularly in patients with treatment-resistant hypertension, as pill burden, complex regimens, comorbid conditions, and medication side effects can all contribute. Tools to assess adherence include prescription fill rates and measuring medication concentration in the blood or urine. While not an exclusion for renal denervation, obtaining this information better informs the shared decision-making process. Interestingly enough, simply informing patients of detected nonadherence can lead to behavioral changes (in up to 80% of patients)11 and reduce BP by up to 46/26 mm Hg.12 Other strategies such as streamlining the regimen, incorporating combination medications, and engaging in dialogue with the patient to understand potential causes of nonadherence—like side effects or cost concerns—are great starting points to attempt to improve adherence.
Advertisement
A 60-year-old female presents for hypertension follow-up. Although she is on four optimally dosed agents (angiotensin-converting enzyme inhibitor, calcium channel blocker, chlorthalidone, and spironolactone), her systolic BP continues to be greater than 140 mm Hg. The physician considers referral for renal denervation.
The history of renal denervation traces back to 1953, when splanchnicectomy (surgical removal of splanchnic nerves) was introduced as a treatment for severe primary hypertension and was shown to be very effective in treating hypertension.13 However, this procedure became obsolete because of significant morbidity, including severe orthostatic hypotension, urinary and fecal incontinence, and erectile dysfunction.
Renal denervation decreases sympathetic nervous signaling between the central nervous system and the kidneys, considered one of many mediators of hypertension and treatment-resistant hypertension.14 Numerous early non-sham-controlled trials of renal denervation demonstrated large BP reductions.15 However, SYMPLICITY HTN-3 (Renal Denervation in Patients With Uncontrolled Hypertension),16 the first pivotal randomized sham-controlled trial, did not meet its primary efficacy end point at 6 months, thereby dampening enthusiasm for this technology. Much has been written about the efficacy results of the SYMPLICITY HTN-3 trial, including critiques of the study design, use of confounding medications, and inconsistent procedural techniques.17 A post hoc analysis derived from patient cohorts showed that there were no significant differences in BP changes between the denervation and sham control group for patients on vasodilators or aldosterone antagonists, although there was a trend for greater change in office systolic BP in patients in the renal denervation group who were receiving beta-blockers and calcium-channel blockers.18
Advertisement
Subsequent randomized sham-controlled trials5,19 addressed these shortcomings and produced compelling evidence supporting the efficacy of renal denervation to lower BP. These seminal trials demonstrated noteworthy, albeit not dramatic, BP reduction in patients with uncontrolled hypertension.
In RADIANCE-HTN TRIO (A Study of the ReCor Medical Paradise System in Clinical Hypertension-Resistance to Triple Medication Pill),5 ultrasound-based renal denervation was compared with a sham procedure in patients with uncontrolled BP despite three or more antihypertensive medications. Renal denervation reduced daytime ambulatory BP more than the sham procedure: −8.0 mm Hg (interquartile range −16.4 to 0) vs −3.0 (interquartile range −10.3 to 1.8). The median between-group difference was −4.5 mm Hg (95% confidence interval [CI] −8.5 to −0.3, adjusted P = .022). The median between-group difference in patients with complete ambulatory BP data was −5.8 mm Hg (95% CI −9.7 to −1.6, adjusted P = .0051).5
The randomized, single-blind, sham-controlled SPYRAL HTN-ON MED (Global Clinical Study of Renal Denervation With the Symplicity Spyral Multi-electrode Renal Denervation System in Patients With Uncontrolled Hypertension on Standard Medical Therapy) expansion trial19 evaluated radiofrequency-based renal denervation in patients with uncontrolled hypertension. The study enrolled 467 patients from multiple countries, 80 of whom were randomized to undergo renal denervation or the sham procedure. At 36 months, the ambulatory systolic BP reduction was −18.7 mm Hg (standard deviation [SD] 12.4) for the renal denervation group (n = 30) and −8.6 mm Hg (SD 14.6) for the sham control group (n = 32), with an adjusted treatment difference of −10.0 mm Hg (95% CI −16.6 to −3.3, P = .0039). Treatment differences between the renal denervation group and sham control group at 36 months were as follows:
Advertisement
Safety concerns surrounding renal denervation are worth addressing. Theoretical concerns include damage to the renal artery from the applied energy, resulting in dissection or de novo stenosis, and contrast-associated nephropathy causing eGFR decline.20,21 Currently, there are no safety signals noted in these trials within the constraints of the populations studied (eGFR > 40 mL/minute/1.73 m2). Longer-term data from Global SYMPLICITY (Global Prospective Registry for Sympathetic Renal Denervation in Selected Indications Through 3 Years) three showed overall reassuring eGFR trends, and new renal artery stenosis (> 70% diameter stenosis) occurred in only 3 (0.1%) of 2,112 patients at risk over a 1-year follow-up period and 4 (0.3%) of 1,345 at risk over 3 years. Notably, the US Food and Drug Administration Circulatory System Devices Panel in August 2023 voted unanimously that both ultrasound-based (Paradise Ultrasound system) and radiofrequency-based (Symplicity Spyral System) renal denervation technologies are safe.6,7
When deciding whether to proceed with renal denervation in patients like the one in scenario 1, clinicians must provide careful education, set realistic expectations, and explore alternative options. Also, renal denervation should only be considered after ruling out pseudoresistance. Patients considering renal denervation must understand that potential BP reduction from denervation is most likely equivalent to that of an additional antihypertensive agent. Aside from having a higher baseline BP and heart rate, no other factors that predict response to renal denervation therapy have been identified.4 Also, there are no head-to-head trials comparing renal denervation and additional pharmacologic interventions.
A 36-year-old female seeks a second opinion regarding the management of hypertension. Diagnosed with hypertension two years ago, she has tried multiple medications with poor tolerance owing to allergic reactions or side effects. Her BP remains uncontrolled, and renal denervation is being considered.
Patients with multiple drug intolerances are candidates for renal denervation. RADIANCE HTN SOLO (A Study of the ReCor Medical Paradise System in Clinical Hypertension)1 examined the use of ultrasound energy–based renal denervation in adult patients ages 18 to 75 with hypertension while off antihypertensive therapy. Patients who underwent renal denervation had a greater reduction in daytime ambulatory systolic BP compared with those who had the sham procedure: −8.5 mm Hg (SD 9.3) vs −2.2 mm Hg (SD 10.0). The difference between groups was −6.3 mm Hg (95% CI −9.4 to −3.1, P = .0001).1
RADIANCE II (A Study of the Recor Medical Paradise System in Stage II Hypertension)22 further evaluated renal denervation in a similar population of adults with previously uncontrolled hypertension on up to 2 antihypertensive medications. The procedure was performed after a 4-week medication washout. Daytime ambulatory systolic BP was significantly reduced with renal denervation (mean −7.9 mm Hg [SD 11.6]) vs sham procedure (mean −1.8 mm Hg [SD 9.5]), with an adjusted difference between groups of −6.3 mm Hg (95% CI −9.3 to −3.2, P < .001). The BP-lowering effect of renal denervation was consistent throughout the 24-hour circadian cycle.22
Similar results were achieved in the SPYRAL HTN-OFF MED (Global Clinical Study of Renal Denervation With the Symplicity Spyral Multi-electrode Renal Denervation System in Patients With Uncontrolled Hypertension in the Absence of Antihypertensive Medications) cohort2 in which patients with hypertension not on any antihypertensive medications achieved a significant drop in BP with renal denervation vs sham, with a difference of −3.9 mm Hg for 24-hour systolic BP and −6.5 mm Hg for in-office systolic BP.
A patient-level pooled analysis of RADIANCE-HTN SOLO, RADIANCE-HTN TRIO, and RADIANCE II revealed that the BP-lowering effect of ultrasound-based renal denervation was consistent across the spectrum of hypertension severity.4 BP reduction effects were shown to be sustained after 3 years in the Global SYMPLICITY registry,3 with the largest BP drop in the subgroups with more severe hypertension. The data illustrate that renal denervation is a reasonable and effective alternative for patients who cannot tolerate or are unable to take medications, even if they do not meet the criteria for true treatment-resistant hypertension.
It is important to note that secondary forms of hypertension represent a contraindication for renal denervation. Before referring the 36-year-old patient in scenario 2 for renal denervation, an in-depth evaluation for secondary causes should be completed. Hypertension treatment in the setting of an underlying secondary cause should be tailored to the underlying pathology. Whether there is a supportive role for renal denervation in select cases is yet to be seen.
Table 1 lists exclusion criteria and characteristics of patients for whom treatment with renal denervation could be appropriate.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d2c2fe7e-1b8e-49df-9cc7-a384e6c2fa44/kidney-ablation-catheter-table)
Table 1. Patient characteristics for potential treatment with renal denervation
Recent studies have demonstrated the efficacy and safety of catheter-based renal artery denervation with radiofrequency or ultrasound energy in reducing blood pressure across the hypertension spectrum, with multiple trials suggesting a significant and sustained reduction in BP. In some studies, BP reduction was sustained for up to 36 months after renal denervation. More data are needed to determine whether attenuating the renal sympathetic nervous system offers end-organ protection beyond BP reduction. Renal denervation may be offered as an alternative or adjunct to pharmacotherapy in patients with apparent treatment-resistant hypertension, multidrug intolerance, or nonadherence. Shared decision-making, including establishing realistic expectations regarding lowering BP, is crucial before proceeding with renal denervation.
Disclosures
Dr Laffin has disclosed being an advisor or review panel participant for research for Arrowhead, AstraZeneca Pharmaceuticals, Crisper Therapeutics, Gordy Health, and LucidAct Health; teaching and speaking for Cardiometabolic Health Congress; Executive Committee member of SURMOUNT MMO Trial for Eli Lilly; consulting for Idorsia Pharmaceuticals Ltd and Medtronic; Executive Committee member in phase 2 trials for Mineralys Therapeutics; and past research relationship with Amgen. Dr Ziada has disclosed teaching and speaking for Abbott Vascular. Dr. Nanjundappa has disclosed teaching and speaking for Abbott, Medtronic, and Phillips Healthcare, consulting for Argon and Phillips Healthcare, and ownership interest (stock, stock options in a publicly owned company) for Zoll. Dr Mehdi has disclosed being an advisor or review panel participant for Fresenius, teaching and speaking for GlaxoSmithKline, and past teaching and speaking for AstraZeneca. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
References
Advertisement
Pediatric urologists lead quality improvement initiative, author systemwide guideline
Fixed-dose single-pill combinations and future therapies
Reproductive urologists publish a contemporary review to guide practice
Two recent cases show favorable pain and cosmesis outcomes
Meta-analysis assesses outcomes in adolescent age vs. mid-adulthood
Proteinuria reduction remains the most important treatment target.
IgA nephropathy is a relatively common autoimmune glomerular disease that can be diagnosed only by biopsy
Oncologic and functional outcomes are promising, but selection is key