Pilot findings show good patient acceptance and safety, early hints of efficacy
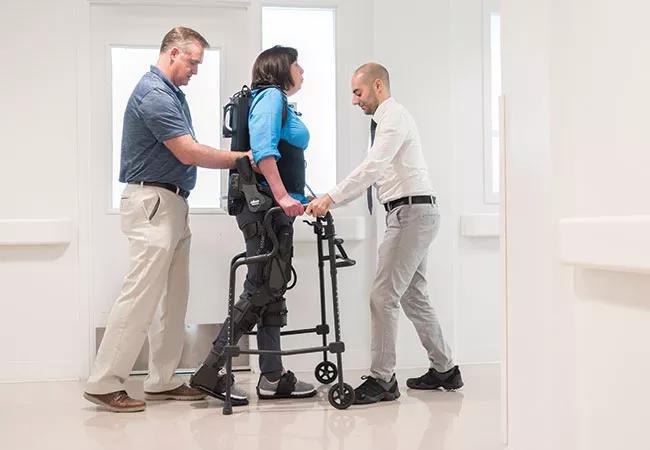
Results of a pilot study by Cleveland Clinic researchers show that a powered exoskeleton is feasible and safe for gait training in rehabilitation of people with multiple sclerosis (MS).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Presented at the virtual 2020 annual conference of the American Congress of Rehabilitation Medicine, the findings — from one of the first assessments of the Ekso GT™ powered exoskeleton in the setting of MS — support future trials of the technology. Use of the adjustable device improved gait speed with no serious adverse effects.
“All five patients with moderate to severe walking limitations completed the training and the exoskeleton was acceptable to them,” says principal investigator Francois Bethoux, MD, Chair of Cleveland Clinic’s Department of Physical Medicine and Rehabilitation. “These first signals from clinical use of the device, in terms of gait improvements and safety, are very encouraging.”
The Ekso GT (shown in photo above) consists of a fitted metal brace supporting the legs, feet and torso. It manipulates the patient’s legs and waist to facilitate standing up, walking on a level surface and sitting down. Battery-powered motors drive the knee and hip joints, with the patient gaining support for balance and body positioning through use of a cane, a walker or crutches.
Rehabilitation training in the five-patient pilot study consisted of three one-hour sessions per week for eight weeks. Sessions consisted of stretching, overground gait training and gait training with the exoskeleton device. A trained physical therapist operated the exoskeleton and monitored the patients to ensure proper balance.
At baseline, all five patients used bilateral support to walk, and 80% used an ankle-foot orthosis. Their mean Expanded Disability Status Scale score was 6.3 ± 0.27. Patients were 60% female and had a mean age of 56.2 and mean disease duration of 25.2 years.
Advertisement
Patients were assessed at baseline, at the end of the eight-week treatment and at four-week follow-up.
Safety evaluation involved assessment for adverse events during or between treatment sessions, with special vigilance for falls and musculoskeletal pain, along with gauging pain severity. Treatment acceptability was assessed with the assistive device subscale of the Quebec User Evaluation of Satisfaction with Assistive Technology, version 2 (QUEST 2.0).
Eight adverse events (pain, skin abrasion, falls between training sessions) were reported by four participants. The average pain score was stable after treatment. Mean QUEST scores ranged from 3.4 to 4.8 on a 5-point scale, with higher scores indicating greater satisfaction with the device.
“The adverse events were mild and reversible,” says Dr. Bethoux. “Patients did have some discomfort with wearing the device, but it was never to the point that they had to discontinue treatment, nor did it threaten their safety.”
Efficacy, evaluated without patients wearing the exoskeleton, was measured in terms of spasticity, hip flexor strength and various gait parameters; the latter were assessed with patients on an overground gait analysis mat and on a treadmill in the Computer Assisted Rehabilitation Environment (CAREN) system.
All patients saw increases from baseline in comfortable gait speed on the CAREN system both at the end of treatment and at four-week follow-up. Step height also improved from baseline at both post-treatment assessments. There was no meaningful change in strength or spasticity in the lower extremities at the end of treatment or follow-up.
Advertisement
Based on the results of this pilot study, the researchers are seeking funding for a phase 2 randomized clinical trial of gait training with and without the exoskeleton in patients with MS. “These pilot findings suggest that comfortable gait speed could be used as a primary outcome measure,” Dr. Bethoux says. If data from a phase 2 trial are positive, the Cleveland Clinic team plans to design a nationwide multicenter trial.
“We’re very interested in looking at whether gait training with the device triggers neuroplasticity in the brain, which would mean that patients realize an enduring benefit from it,” notes Dr. Bethoux. “That is really the future, but we need strong evidence to guide decision-making about use of the exoskeleton in MS, as we have with medications.” Currently, the Ekso GT exoskeleton is not indicated for use in MS.
While research continues, trained physical therapists in Cleveland Clinic’s Mellen Center for Multiple Sclerosis Treatment and Research have started using the exoskeleton for gait training in patients with MS who are interested in robot-assisted rehabilitation. “We are glad to make this type of equipment available to some of our patients,” says Dr. Bethoux, who serves as the Mellen Center’s Director of Rehabilitation Services, “and the evidence generated from our research will help guide its clinical use.”
Advertisement
Advertisement

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

An expert’s take on evolving challenges, treatments and responsibilities through early adulthood

Comorbidities and medical complexity underlie far more deaths than SUDEP does

Novel Cleveland Clinic project is fueled by a $1 million NIH grant

Tool helps patients understand when to ask for help