Web-based self-selection tool aims to achieve long-sought nonprescription statin access

Only about half of individuals who are eligible for statin therapy actually receive statin treatment, according to recent registry data. To take fuller advantage of statins’ benefits for primary and secondary cardiovascular prevention, some public health advocates have proposed making low-dose statin therapy available without a prescription. However, five separate efforts to obtain U.S. regulatory approval for over-the-counter statins have failed to date. The main reason for these failures was an inability to show that consumers could appropriately self-select for treatment.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Now a new study has shown that using a novel approach — technology-assisted self-selection — to qualify consumers for nonprescription statin treatment was successful in ensuring that a high percentage of ineligible consumers were denied access and only those at an appropriate level of cardiovascular risk were deemed eligible. The study used a web application to evaluate whether U.S. consumers can appropriately self-select for treatment with rosuvastatin 5 mg once daily by measuring agreement between consumer assessment and clinician assessment of treatment eligibility. Its results were published in the Journal of the American College of Cardiology (2021;78[11]:1114-1123).
“By demonstrating a high level of concordance between consumer assessment and clinician assessment of eligibility for statin treatment, our study demonstrates this new technology-assisted approach can overcome traditional barriers and may be a good model for achieving the goal of safe nonprescription access to statins for appropriate individuals,” says lead author Steven Nissen, MD, Chief Academic Officer of Cleveland Clinic’s Miller Family Heart, Vascular & Thoracic Institute.
The web application used in the study was programmed to reflect the 2018 American College of Cardiology/American Heart Association (ACC/AHA) cholesterol treatment guidelines for moderate-intensity statin therapy, as well as the proposed warnings and precautions for nonprescription rosuvastatin (“drug facts” label), using the ACC/AHA risk calculator pooled cohort equations for calculating cardiovascular risk.
Advertisement
The study’s 500 participants were recruited from the general population through advertising. Enrollees had to be at least 20 years old, able to speak and read English and not employed in healthcare. Enrollment was regulated to preclude excessive numbers of young and old individuals who would be deemed statin-ineligible solely due to age. Additionally, the design required at least 16% of enrollees to have limited literacy to reflect real-world literacy levels.
Study participants accessed the web-based application at home or a location of their choice, responding to questions about their medical history, medication use, cholesterol levels (total, LDL, HDL), triglycerides, blood pressure and, if needed, waist circumference, high-sensitivity C-reactive protein and coronary calcium score.
Based on their responses, participants were assigned one of three self-selection outcomes:
Participants were considered ineligible for nonprescription therapy if their cardiovascular risk score was < 5% or > 20%, if they had a risk between 5% and 7.5% without a risk-enhancing factor or if they had a contraindication to rosuvastatin.
After completing the online self-selection, participants were directed to a research site for a scheduled visit and told to bring verification of their laboratory values and blood pressure as reported through the web application. If verification wasn’t available, these measures could be taken on site. The information collected at the site visit was sent to a telemedicine clinician, who conducted an independent medical evaluation and used this information, together with the verified lab and blood pressure data, to complete a technology-assisted assessment via the same web application used by the participant. Clinicians were blinded to the web entries made by the participant, and both participants and clinicians were blinded to the self-selection outcome participants were assigned by the web application.
Advertisement
The primary endpoint was the proportion of participants whose self-selection outcome for nonprescription rosuvastatin matched that of the clinician’s technology-assisted assessment. The secondary endpoint was the percentage of participants who had correct entries for all questions, to identify which questions were most apt to lead to errors.
Final analysis of all data was conducted by the Cleveland Clinic Coordinating Center for Clinical Research (C5Research).
Among 1,563 volunteers assessed for study inclusion, 1,063 were sent a link to the web-based application. Of these, 563 did not schedule an on-site visit, leaving 500 with both a self-selection assessment and a clinician assessment. These 500, who made up the final study cohort, had a mean age of 59.2 years and were 62.2% female and predominantly White (61.0%) or Black (33.2%). Forty-five percent of the cohort had graduated from college or a technical school. Notably, the 563 participants who did not schedule on-site visits were demographically similar to this cohort.
Key results were as follows:
Advertisement
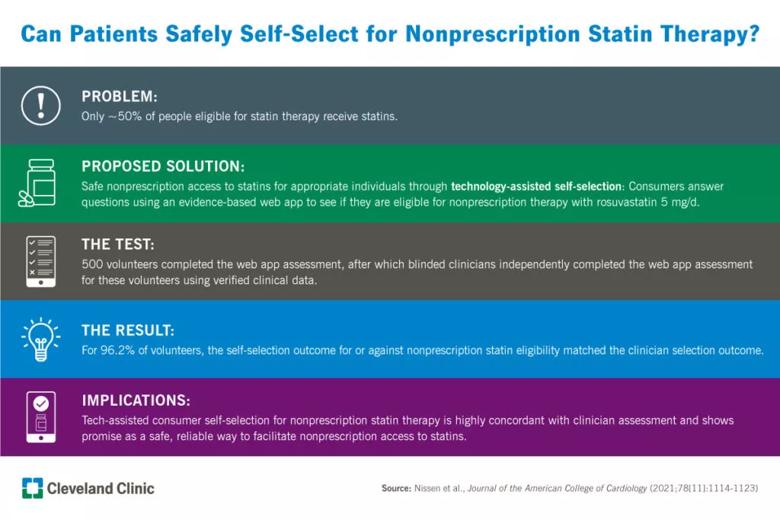
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/3cf8650c-a753-4d7d-9c4f-f7bf1f56e26f/21-HVI-2375209-visual-abstract-inset-1024x683_jpg)
“These findings show that using a technology-assisted self-selection tool for nonprescription statin therapy resulted in consumer self-selection that substantially agreed with clinician selection,” Dr. Nissen observes.
Additionally, a similar rate of concordant outcomes — 96.4% — was observed within the subgroup of participants with limited literacy (n = 83). “This is important since consumers with limited literacy may have reduced access to the healthcare system and therefore may benefit more from nonprescription access to statin therapy,” Dr. Nissen says.
He points out that it was expected that a large majority of study participants (91.6%) would be deemed ineligible for nonprescription statin therapy because the study was designed to enroll a broad sampling of the general population. Such a design is necessary because a failure to prevent treatment access for ineligible consumers was a critical shortcoming of earlier attempts to provide nonprescription access to statins.
Dr. Nissen notes that this study’s small number of incorrect self-selections for therapy — just three cases out of 500 — is impressive in light of those prior efforts. “Incorrect self-selection can raise safety concerns by permitting access to medication among consumers who are not likely to benefit or who may be at risk for adverse effects,” he says.
Notably, even the three participants in this study whose self-selection for therapy was discordant with the clinician assessment against therapy would have ended up benefiting from therapy after erroneous participant responses were subsequently identified.
Advertisement
Prior over-the-counter statin programs’ lack of success can be explained by two key factors, the study authors write:
In contrast, they note, the web application in this technology-assisted approach supplemented the drug facts label with current cholesterol treatment guidelines and with the ACC/AHA risk calculator for cardiovascular risk. Additionally, rosuvastatin 5 mg/day was chosen for this program because it has greater efficacy in LDL cholesterol reduction than other statin entry doses while also demonstrating “a safety profile appropriate for use in a nonprescription setting,” they write.
The web-based application used in the study was developed under the regulatory guidance for Software as a Medical Device. If it receives FDA approval, its proposed development program would allow consumers who are deemed eligible after completing the web application to make an online purchase of rosuvastatin 5 mg without a prescription. The medication would be shipped directly to the consumer and would not be available for over-the-counter purchase in pharmacies or stores. Renewal reminders would be emailed to purchasers as their medication supply dwindled, directing them back to the web application to take a brief health reassessment to confirm their continued eligibility.
The study authors note that future investigations are needed to enroll consumers with a high likelihood of eligibility for nonprescription rosuvastatin to learn whether this technology-assisted approach can improve appropriate selection of therapy candidates and to evaluate their adherence to treatment and long-term clinical outcomes.
The study was funded by AstraZeneca Pharmaceuticals.
Advertisement

Cleveland Clinic’s new dedicated program offers nuanced care for a newly recognized cardiovascular risk factor

Scenarios where experience-based management nuance can matter most

Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic