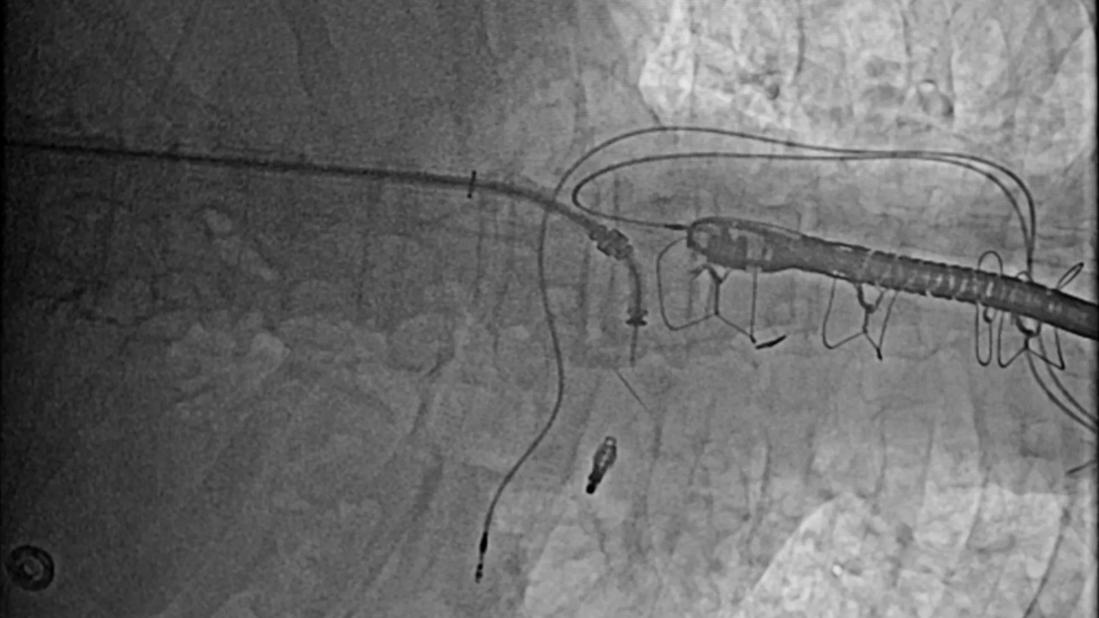
Open-heart surgery has long been the standard of care for most patients with valvular disease, including aortic stenosis. More recently, however, minimally invasive procedures, such as transcatheter aortic valve replacement (TAVR) and the MitraClip™ procedure, have emerged as safe and effective alternatives for patients with advanced and complex valvular disease.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A multitude of studies have shown TAVR to be a safe and effective alternative to traditional valve replacement surgery. TAVR is available for patients of all risk levels with severe aortic stenosis, including those with degenerative aortic bioprosthesis stenosis or regurgitation.
“The TAVR procedure has revolutionized the way we manage patients with valvular heart disease,” says Emad Hakemi, MD, an interventional cardiologist with Cleveland Clinic Florida’s Structural Heart Disease Program and Multidisciplinary Valve Clinic.
The heart valve specialists at Cleveland Clinic Florida routinely perform TAVR using a minimalistic approach. With the patient under local anesthesia and mild sedation, a new valve is inserted within the diseased valve through percutaneous transfemoral access. This approach provides patients with an expedited recovery with early discharge at one to two days.
The transcatheter mitral valve edge-to-edge repair procedure has evolved to become an important solution for patients with mitral regurgitation for whom medical therapy fails. Initially approved for non-surgical candidate patients with severe degenerative mitral regurgitation (MR), this therapy is now also available for those with functional mitral regurgitation.
“The multicenter randomized COAPT trial of patients with severe functional MR demonstrated that this therapy resulted in marked improvement in survival, hospitalizations and heart failure class,” explains Dr. Hakemi.
MitraClip™ is presently the only FDA-approved transcatheter therapy for this indication. The procedure is performed under general anesthesia using 3D transesophageal echocardiography. Following transeptal access, the MitraClip is delivered to create a double orifice mitral valve that reduces the severity of the mitral regurgitation.
Advertisement
The use of balloon-expandable transcatheter valve bioprosthesis has been broadened to cover patients with degenerated mitral valve bioprosthesis or failing mitral valve repair. This groundbreaking minimally invasive procedure is done under general anesthesia, and the femoral vein is used to advance a transcatheter bioprosthesis through the inter-atrial septum into the left atrial cavity. From there, the valve is positioned and deployed within the degenerated mitral valve bioprosthesis or mitral valve ring. “Patients benefit from the less invasive nature of this therapy with a faster recovery in comparison to the extensive surgery that had traditionally been performed, which required repeat sternotomy for redo mitral valve replacement,” Dr. Hakemi notes.
Advertisement
Advertisement

Nonthermal technique reduces bleeding and perforation risk

Standardizing a minimally invasive approach for Barrett’s Esophagus and Esophageal Cancer

PSMA-targeted therapy for metastatic prostate cancer now offered at Cleveland Clinic Weston Hospital

Nationally recognized urologic oncologist offers vision for growth, innovation, and excellence

Noninvasive modality gains ground in United States for patients with early-to-moderate disease

Cleveland Clinic Weston Hospital’s collaborative model elevates care for complex lung diseases

Interventional pulmonologists at Cleveland Clinic Indian River Hospital use robotic technology to reach small peripheral lung nodules

Trained in the use of multiple focal therapies for prostate cancer, Dr. Jamil Syed recommends HIFU for certain patients with intermediate-risk prostate cancer, especially individuals with small, well-defined tumors localized to the lateral and posterior regions of the gland.