Findings from a recent randomized trial investigating mepolizumab
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A 55-year-old female was diagnosed with eosinophilic granulomatosis with polyangiitis (Churg-Strauss, EGPA) five years ago. Her features included an eight-year history of asthma, sinus congestion with nasal polyps, eosinophilia, cutaneous small-vessel vasculitis with abundant tissue eosinophils, and a sensory neuropathy. Testing for antineutrophil cytoplasmic antibodies (ANCA) was negative, and echocardiogram was normal. She was initially treated with prednisone and methotrexate which was switched to azathioprine for intolerance. Although this treatment provided good control of the vasculitic features, she continued to have recurrent asthma despite optimized inhaled regimens requiring frequent glucocorticoid bursts and prednisone > 10 mg/day impacting her quality of life. She has questions today about whether there are other treatment options.
EGPA is characterized by allergic, eosinophilic and vasculitic manifestations. Although it is within the family of ANCA-associated vasculitis, it is phenotypically and therapeutically different from granulomatosis with polyangiitis (Wegener’s, GPA) and microscopic polyangiitis (MPA).
A similarity in treatment between EGPA, GPA and MPA is the use of glucocorticoids and cyclophosphamide (CYC) in patients with life-threatening vasculitic manifestations. In EGPA, an important manifestation where this regimen is commonly applied is in active cardiac involvement which can be associated with poor outcomes. When CYC is used, it is typically given for three to six months, followed by a maintenance approach with azathioprine, methotrexate or mycophenolate.
Advertisement
While rituximab has been proven to be as effective as CYC in GPA and MPA, there remains a limited body of published data with rituximab in EGPA. Although rituximab has been used in EGPA for patients who cannot take CYC or have mild-to-moderate vasculitic features despite other conventional immunosuppressives, there is currently insufficient evidence to support its use in life-threatening disease in patients where CYC would be an option.
A unique aspect of management in EGPA is the treatment of the allergic and eosinophilic sinus and asthmatic features. For some patients with EGPA, these remain the most problematic manifestations that may limit the ability to taper glucocorticoids to acceptable dosages. For such features, it is important to optimize the use of inhaled glucocorticoids and bronchodilators, working in close collaboration with an asthma specialist. Beyond glucocorticoids, the effectiveness of other conventional immunosuppressive agents for the asthmatic features is variable between patients. There has been insufficient information to establish whether rituximab provides benefit for these features.
Mepolizumab is a monoclonal antibody that binds to interleukin-5 (IL-5) and prevents interaction with its receptor on the eosinophil surface. IL-5 plays an integral role in the maturation, proliferation and differentiation of eosinophils, which has made this an intriguing agent to investigate in eosinophilic diseases. In 2015, mepolizumab was approved by the FDA for the treatment of severe eosinophilic asthma in patients over the age of 12.
Advertisement
A multicenter, international, double-blind randomized trial was recently conducted to examine the safety and efficacy of mepolizumab in EGPA following encouraging results in pilot studies. The trial enrolled 136 patients at 31 sites. Patients had to be 18 years or older with non-life-threatening relapsing or refractory EGPA on a stable dose of prednisone/prednisolone 7.5-50 mg/day for at least four weeks.
Participants underwent blinded randomization to receive mepolizumab 300 mg subcutaneously once a month or placebo for 52 weeks. The glucocorticoid dose had to remain stable between randomization and week four following which this could be tapered at the investigator’s discretion using a standardized reduction schedule. Patients who were on a maintenance immunosuppressive continued this agent at the same dose. In this study, remission was defined as a BVAS = 0 with a prednisone/prednisolone dose of < 4.0 mg/day with relapse being active vasculitis BVAS > 0, active asthma or active nasal/sinus disease leading to an increase in prednisone/prednisolone > 4.0 mg/day, addition of another immunosuppressive or hospitalization.
In terms of efficacy, mepolizumab resulted in more weeks of remission with 28 percent having > 24 weeks of accrued remission compared to 3 percent in those who received placebo (P < 0.001). It also led to a higher percentage of patients in remission at both weeks 36 and 48 compared to placebo (32 vs 3 percent, P < 0.001). Overall, remission did not occur in 47 percent of those who received mepolizumab as compared to 81 percent who received placebo. An average dose of prednisone < 4.0 mg/day was reached during weeks 48-52 in 44 percent of those receiving mepolizumab compared to 7 percent for placebo (P < 0.001). In terms of safety, there was no significant difference in the percentage of adverse events between those who received mepolizumab and placebo with the most common events being headache, nasopharyngitis, arthralgias and upper respiratory tract infection.
Advertisement
The role of mepolizumab in the treatment of EGPA remains yet to be defined (mepolizumab is not FDA approved for EGPA). In the EGPA randomized trial, mepolizumab resulted in more weeks in remission with a higher percentage of patients in remission than did those who received placebo, and showed efficacy for all primary and secondary endpoints. However, only half of the patients who received mepolizumab had a protocol-defined remission.
As patients with life-threatening EGPA were excluded from the mepolizumab trial, mepolizumab should not be used in this setting. In the EGPA trial, mepolizumab was given at a dose of 300 mg every 4 weeks which is higher than the FDA approved asthma dosage of 100 mg every 4 weeks. Although patients with mild-to-moderate vasculitis were eligible for this study, the efficacy of a lower dose for managing vasculitic manifestations is unknown such that use of conventional immunosuppressive agents remains the best evidence-based approach at this time. Should mepolizumab become FDA approved for EGPA and available at 300 mg every 4 weeks, consideration of use for mild-to-moderate vasculitis should continue to be viewed with caution on a case-by-case basis examining the nature of the disease features, the past disease history, and whether there have been relapses or contraindications to conventional immunosuppressive agents.
In our patient with relapsing eosinophilic asthma who is unable to taper glucocorticoids to an acceptable level, mepolizumab would be a consideration given the proven experience in asthma combined with the encouraging experience in the EGPA trial. In such a setting, careful ongoing monitoring for emergence of new EGPA features would remain important.
Advertisement
The investigation of mepolizumab has provided valuable information in EGPA not only in demonstrating therapeutic efficacy but also for the ability to conduct blinded, randomized trials in this rare disease. Although there remain ongoing questions to be answered regarding the role of mepolizumab, this body of work provides exciting evidence of what lies ahead in the understanding and management of this complex vasculitic disease.
Dr. Langford is Director of the Center for Vasculitis Care and Research as well as Vice Chair for Research, Department of Rheumatic and Immunologic Diseases.
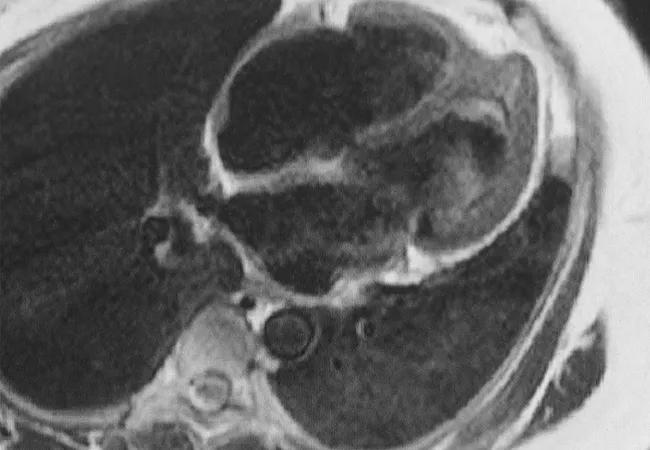
Feature image: Cardiac MRI demonstrating complications from heart involvement in EGPA (Churg-Strauss). This patient has endomyocardial fibrosis at the left ventricular apex with a large apical thrombus. Image and caption reprinted with permission from Elsevier.
Disclosure: Dr. Langford and Cleveland Clinic were members of the EGPA mepolizumab study team participating in the clinical trial funded by GlaxoSmithKline and the linked mechanistic studies funded by the National Institute of Allergy and Infectious Diseases.
Advertisement

A conversation with Leonard Calabrese, DO

The case for continued vigilance, counseling and antivirals

High fevers, diffuse rashes pointed to an unexpected diagnosis

No-cost learning and CME credit are part of this webcast series

Summit broadens understanding of new therapies and disease management

Program empowers users with PsA to take charge of their mental well being

Nitric oxide plays a key role in vascular physiology

CAR T-cell therapy may offer reason for optimism that those with SLE can experience improvement in quality of life.