A case study in diagnosing and managing unexplained acute flank pain
By Mohammad A. Sohail, MD; John Sedor, MD; Lee Kirksey, MD, MBA; Steven C. Blaha, MD; and Heather Hofmann, MD, FACP
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Editor’s note: This open-access article originally appeared in the Cleveland Clinic Journal of Medicine (2022;89:465-471).
A 45-year-old woman with a history of hypertension and ischemic stroke at age 26 presented to the emergency department with right-sided flank pain, which had started two days earlier. The pain was constant, sharp, nonradiating, worse with movement and coughing, and severe enough to limit ambulation and induce nausea and vomiting. She said she had not recently been injured.
She also reported that for five years she has had intermittent left-sided low back pain that radiates to her left foot. Earlier imaging of the lumbar spine showed spinal stenosis and L1-L2 vertebral disc extrusion.
Her hypertension was diagnosed 20 years ago, and she said she adhered to her antihypertensive regimen of amlodipine 10 mg and hydrochlorothiazide 25 mg daily. She previously took lisinopril, but it was discontinued because it made her cough.
The etiology of her prior stroke was undetermined. The patient had never been diagnosed with diabetes mellitus, atrial fibrillation, coronary artery disease, or peripheral vascular disease, but had been actively smoking cigarettes since age 30 (10 pack-years). She reported no residual deficits from her stroke.
Her blood pressure was 196/106 mm Hg, pulse regular at 75 beats per minute, temperature 37.5°C (99.5°F), and oxygen saturation between 95% and 100% while breathing room air. She did not appear to be in distress. Pulses were slightly diminished throughout her body, rated 2+ on a scale of 0 to 4+, and there was no carotid bruit. Heart sounds S1 and S2 were normal, there were no murmurs, and the point of maximal impulse was not displaced.
Advertisement
Findings on respiratory and abdominal examinations were normal. Her right lumbar region was tender to palpation, but not the costovertebral angle. Her extremities were without edema, joints were without synovitis, and ranges of motion were within normal limits. The cranial nerves were intact, and sensory and motor examinations had no focal findings.
Laboratory test results
Results of blood testing were as follows:
Urinalysis with microscopic examination showed the following:
Results of additional testing were as follows:
Advertisement
Comment. These laboratory results reveal hypokalemia, normal renal function, and elevated renin. The urine toxicology screen and aldosterone, renin, and cortisol levels were obtained as part of a secondary hypertension workup. The hypokalemia was attributed to elevated renin.
A new finding on imaging
The patient’s pain was initially believed to be musculoskeletal, so she was given acetaminophen, cyclobenzaprine, and a lidocaine patch. However, this gave only minimal relief, prompting computed tomography (CT) of the abdomen and pelvis with intravenous contrast to look for other causes.
CT showed that the right kidney measured only 6.2 cm in length, while the left kidney was 10.2 cm. This was new: one year earlier, the right kidney had measured 10.1 cm (Figure 1). There were no acute radiographic abnormalities in the lumbar spine or visceral organs. Even though the study was not tailored to evaluate the renal arteries, it showed the right renal artery to be very narrow in caliber.
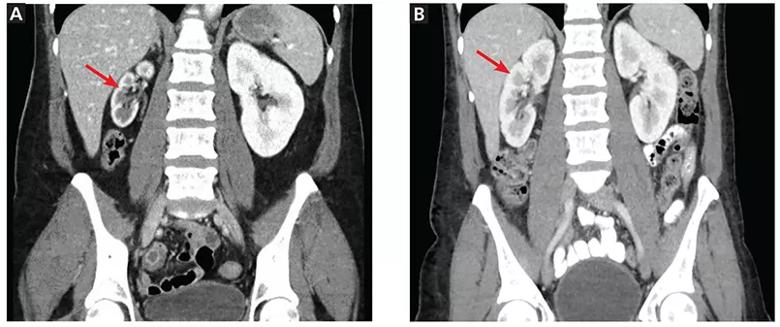
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/62578a65-bbe4-4880-a209-4e6c78c2fb37/22-URL-3418659-CQD-Inset_jpg)
Figure 1. (A) At presentation, computed tomography showed the right kidney (arrow) measuring only 6.2 cm. (B) One year earlier, the right kidney (arrow) had measured 10.1 cm.
The patient’s history of hypertension along with this new radiographic finding of right kidney atrophy with small caliber of the right renal artery raised suspicion for renal artery stenosis, and the patient was admitted for further evaluation.
1. All of the following features of our patient’s case raise suspicion of fibromuscular dysplasia, except which one?
Advertisement
All of the above clinical characteristics, except for the patient’s preserved eGFR, inactive urine sediment, and non-nephrotic-range proteinuria, raised our suspicion for renal artery stenosis due to fibromuscular dysplasia. This is because several other causes of secondary hypertension, including primary aldosteronism, atherosclerotic renal artery stenosis, pheochromocytoma, hypercortisolism, hypothyroidism, and primary hyperparathyroidism, are also characterized by preserved kidney function and a relatively bland urinalysis.
The narrowing of the patient’s right renal artery could have been due to several pathologic processes, including stenosis or dissection. Renal artery stenosis and dissection may have similar clinical manifestations including progressive renovascular hypertension, changes in kidney function, and symptoms of kidney infarction such as flank pain.1 Therefore, a definitive diagnosis cannot be discerned until additional imaging studies are performed.2
Atherosclerotic disease is the most common cause of renal artery stenosis, accounting for up to 90% of all cases.3
Fibromuscular dysplasia, the second most common cause, is a noninflammatory vascular disorder affecting multiple arterial beds.4 In addition to stenosis, it can cause aneurysm and dissection.5 It mainly affects the renal arteries and extracranial cerebrovascular arteries, and nearly half of patients may have disease in more than 1 site.6
Fibromuscular dysplasia commonly manifests as hypertension, flank pain, headache, tinnitus, neck pain, and stroke, reflecting involvement of the aforementioned arteries.7,8 Transient ischemic attack and stroke are due to complications such as aneurysm rupture, dissection, or cerebral thromboembolism.9 Our patient’s history of ischemic stroke at age 26 without traditional risk factors for atherosclerosis aside from hypertension, and in the absence of atrial fibrillation, points to fibromuscular dysplasia.
Advertisement
The patient’s early onset of hypertension made us suspect renovascular hypertension rather than primary hypertension. Renal fibromuscular dysplasia causes renovascular hypertension through decreased renal perfusion leading to activation of the renin-angiotensin-aldosterone system with subsequent sodium and water retention. These effects are prominent in patients with a solitary functioning kidney or with bilateral involvement. There are also downstream effects: increased intrarenal prostaglandin concentrations, increased sympathetic nervous system activity, and decreased nitric oxide production. Flank pain, renal infarction, and eventually, atrophic kidneys can manifest later in the disease with development of active renal ischemia, aneurysm rupture, or renal artery dissection.
In adults, kidney size is clinically less relevant than kidney function. Atrophy is usually defined as a reduction in kidney length of more than 1 cm,10,11 and this patient’s right kidney had decreased in size by almost 4 cm, to less than 7 cm. Although our patient does have some atherosclerotic risk factors (hypertension and tobacco use), her history of ischemic stroke at age 26 expanded the differential diagnosis to include nonatherosclerotic causes of kidney atrophy, especially fibromuscular dysplasia. In addition, smoking increases the risk of developing atherosclerosis as well as fibromuscular dysplasia.12
2. What would be the best initial diagnostic test for renal artery stenosis in this patient?
Noninvasive diagnostic testing for renal artery stenosis with CT angiography, magnetic resonance angiography, or duplex ultrasonography should be pursued if clinical findings suggest the patient’s hypertension may be due to renovascular disease.
CT angiography and magnetic resonance angiography are highly sensitive and specific for diagnosing atherosclerotic renal artery stenosis and are preferred over duplex ultrasonography, especially in patients with normal renal function, as they involve the use of contrast media.13,14 Duplex ultrasonography has lower spatial resolution and is highly operator-dependent.13,14
However, we suspected our patient had fibromuscular dysplasia, which frequently involves distal renal arterial segments. Although CT angiography has respectable diagnostic accuracy for fibromuscular dysplasia involving the main renal arteries,15 both CT angiography and magnetic resonance angiography have low sensitivity, ranging from 22% to 28%, for detecting distal disease in the intrarenal portion of the renal artery.16,17 Duplex ultrasonography can detect increased blood flow velocities and hemodynamically significant stenotic lesions in the middle and distal portions of the renal artery, which are frequently involved in fibromuscular dysplasia.18
Invasive diagnostic procedures such as digital subtraction angiography are warranted if a patient’s clinical characteristics are such that the benefits of a potential revascularization of a stenotic lesion would outweigh the risks of undertaking the procedure (see “Indications for renal revascularization” section, below).19 Digital subtraction angiography is the standard and has the highest spatial resolution. It is less frequently performed as the initial test, but when clinical suspicion is high and the results of noninvasive tests are inconclusive, it is recommended to establish the diagnosis of renal artery stenosis.20 In practice, it may also be performed once the diagnosis of renal artery stenosis is established by a noninvasive imaging test and revascularization is planned, after weighing the potential risks and benefits.21
For patients with diminished renal function that prohibits the use of radiocontrast agents, carbon dioxide angiography can be performed for diagnosis, treatment, or both,22 although its image quality is sometimes suboptimal.
In our patient’s case, we strongly suspected renal artery stenosis, the potential benefit of revascularization was initially unclear, and the patient was at a high-volume medical center with expertise in duplex ultrasonography. For these reasons, duplex ultrasonography was done. However, since duplex ultrasonography is operator-dependent, had the patient not been at a facility with expertise in duplex ultrasonography, CT angiography would have been a reasonable alternative.
Renal artery duplex ultrasonography suggested a hemodynamically significant diffuse stenosis of the right renal artery with normal flow on the left.
Since imaging did not reveal a characteristic focal or multifocal stenotic lesion, the patient’s renal artery stenosis could not be definitively attributed to fibromuscular dysplasia. Nephrologists were consulted to guide antihypertensive therapy, and vascular surgeons were consulted regarding whether the patient could benefit from a revascularization procedure for renal artery stenosis.
We decided to perform split-function testing to elucidate the contribution of the patient’s right kidney to her total GFR. This involves injection of radioactive technetium 99m diethylenetriamine pentaacetate and subsequent imaging as the substance is excreted. Therefore, we did not yet start treatment with an inhibitor of the renin-angiotensin-aldosterone system, which could have affected renal perfusion and confounded the results of the study. Instead, the patient’s blood pressure was controlled with amlodipine 10 mg daily and hydralazine 25 mg twice a day. Split-function testing demonstrated that her left kidney was doing almost all the work, with the right kidney contributing only 10% of the total.
3. Which of the following is an appropriate indication for renal revascularization for renal artery stenosis due to fibromuscular dysplasia?
Patients with renal fibromuscular dysplasia can undergo renal artery revascularization if the procedure is necessary to prevent progressive renal loss or if it is likely to cure their hypertension or at least improve control of blood pressure. Patients are most likely to benefit if they are young, have focal fibromuscular dysplasia, do not have underlying atherosclerotic vascular disease, have resistant recent-onset hypertension despite taking multiple antihypertensive agents, or have unexplained progressive renal insufficiency.
Managing hypertension in fibromuscular dysplasia
In patients with renal fibromuscular dysplasia, the primary goal of revascularization is to cure difficult-to-control hypertension. This is a more reasonable goal for this patient group than for those who have renal artery stenosis that is due to atherosclerosis.
A meta-analysis23 of eight randomized trials in patients with atherosclerotic unilateral renal artery stenosis found no benefit in adding percutaneous transluminal renal angioplasty to antihypertensive therapy in terms of relevant clinical outcomes, including end-stage kidney disease, major adverse cardiovascular events, or death.
Further, very few patients who have unilateral atherosclerotic renal artery stenosis are cured of their hypertension by undergoing percutaneous renal angioplasty (with cure defined as normalization of blood pressure and stopping antihypertensive therapy).24,25 Rates are higher in those with unilateral fibromuscular dysplasia,26,27 45% in one study.26
To date, however, no randomized trials have been done to study percutaneous transluminal renal angioplasty in patients with renal fibromuscular dysplasia. The differences in pathophysiology between atherosclerosis and fibromuscular dysplasia make it difficult to draw conclusions about this treatment for renal fibromuscular dysplasia from data in patients with atherosclerotic renal artery stenosis.
Focal vs multifocal fibromuscular dysplasia
Fibromuscular dysplasia differs according to whether it is focal or multifocal.
Carbon dioxide digital subtraction angiography, though not necessary for the initial diagnosis of renal fibromuscular dysplasia, may help to classify it as focal or multifocal. Using this imaging, multifocal fibromuscular dysplasia has a beaded appearance, whereas focal fibromuscular dysplasia appears as concentric, smooth, band-like focal or tubular stenosis. It is suggested that young hypertensive patients with focal fibromuscular dysplasia should undergo revascularization, since many of them have resistant hypertension and are at distinct risk of kidney atrophy.28
On the other hand, multifocal fibromuscular dysplasia usually presents as hypertension that can typically be controlled on an average of 2 antihypertensive medications.28 Studies have shown that there is a lower likelihood of hypertension cure after revascularization in patients with multifocal fibromuscular dysplasia, as well as those with longer duration of hypertension and underlying chronic kidney disease.26,29 The same can be said for extrarenal fibromuscular dysplasia: renal revascularization to cure hypertension in fibromuscular dysplasia is less successful in patients with systemic involvement of multiple vascular beds than in patients with isolated renal fibromuscular dysplasia.30
Since the primary goal of revascularization in patients with fibromuscular dysplasia is to cure difficult-to-control hypertension, revascularization may have a minimal role for patients with multifocal fibromuscular dysplasia.
In adults with focal fibromuscular dysplasia, progressive renal insufficiency may occur secondary to either stenosis from intimal fibroplasia or renal artery dissection. Revascularization is indicated in focal fibromuscular dysplasia to prevent kidney atrophy and chronic kidney disease.6
Duration of hypertension is also something to consider. One study suggested that the blood pressure is unlikely to fall after renal revascularization if the duration of renovascular hypertension has been more than five years.31
Balloon angioplasty vs stenting
For revascularization, balloon angioplasty is preferred over stenting. There is some evidence that drug-coated balloon therapy may provide a longer-lasting benefit. Revascularization of fibromuscular dysplasia with ex vivo reconstruction and auto-transplantation is an appropriate consideration for patients with distal renal artery stenosis or stenosis involving the segmental level vessel. Nephrectomy may be considered for patients with an atrophic kidney and hypertension that is refractory to pharmacologic management.
ACE inhibitors or ARBs preferred
Hypertension in patients with renal fibromuscular dysplasia is caused by a reduction in kidney perfusion and the subsequent activation of the renin-angiotensin-aldosterone system. This dictates that the initial antihypertensive therapy for these patients be either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB).32 When kidney perfusion is low, angiotensin II mediates a preferential increase in the resistance of the efferent arteriole. Starting an ACE inhibitor or an ARB blunts this normal autoregulatory response and causes a hemodynamically mediated decline in the GFR.33 However, despite the initial reduction in filtration in the stenotic kidney, the total GFR is usually maintained due to an approximately equivalent increase in filtration in the contralateral kidney. This is because blocking the vasoconstrictive effect of angiotensin II eventually decreases renal vascular resistance and ultimately preserves renal blood flow.34
Our patient had an atrophic right kidney associated with a right renal artery stenotic lesion with lateralization of renal function on split-function testing. She may have been a candidate for percutaneous transluminal renal angioplasty to preserve kidney function if her condition had been discovered earlier, before the kidney had atrophied.
Additional components in this patient’s case that we considered included the following: she had long-standing hypertension; she had not yet received optimal antihypertensive therapy; she did not have intolerance to these medications; and she did not have refractory heart failure.
We explained the risks and benefits of revascularization, with assistance from a vascular surgeon. The patient’s atrophic right kidney and diffuse stenosis of the right renal artery precluded her from undergoing revascularization. Instead, we initiated renin-angiotensin-aldosterone system blockade with an ARB. Amlodipine and hydrochlorothiazide were maintained for additional blood pressure control while hydralazine was discontinued.
The patient’s right-sided flank pain eventually improved during her hospitalization with conservative management with acetaminophen in combination with muscle relaxants. As mentioned previously, flank pain may be a manifestation of active renal ischemia in patients with renal artery stenosis or dissection prior to the development of kidney atrophy. However, this was less likely to be the cause of flank pain in our patient since there was no evidence of renal infarction on imaging, the right kidney was already atrophied indicating chronic rather than active renal ischemia, and her flank pain improved with conservative measures alone.
The patient was subsequently discharged home on an antihypertensive regimen consisting of losartan, amlodipine and hydrochlorothiazide. The patient was provided with a list of warning signs for uncontrolled hypertension that would require immediate medical attention. Multidisciplinary follow-up appointments with specialists in vascular medicine and nephrology were arranged. Treatment goals included continued risk-factor reduction to maintain the function of her left kidney.
References
1. Plouin PF, Perdu J, La Batide-Alanore A, Boutouyrie P, Gimenez-Roqueplo AP, Jeunemaitre X. Fibromuscular dysplasia. Orphanet J Rare Dis 2007; 2:28. doi:10.1186/1750-1172-2-28
2. Ramamoorthy SL, Vasquez JC, Taft PM, McGinn RF, Hye RJ. Nonoperative management of acute spontaneous renal artery dissection. Ann Vasc Surg 2002; 16(2):157–162. doi:10.1007/s10016-001-0154-0
3. Shivapour DM, Erwin P, Kim ESH. Epidemiology of fibromuscular dysplasia: a review of the literature. Vasc Med 2016; 21(4):376–381. doi:10.1177/1358863X16637913
4. Gornik HL, Persu A, Adlam D, et al. First international consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med 2019; 24(2):164–189. doi:10.1177/1358863X18821816
5. Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation 2014; 129(9):1048–1078. doi:10.1161/01.cir.0000442577.96802.8c
6. Plouin PF, Baguet JP, Thony F, et al. High prevalence of multiple arterial bed lesions in patients with fibromuscular dysplasia: the AR-CADIA registry (Assessment of Renal and Cervical Artery Dysplasia). Hypertension 2017; 70(3):652–658. doi:10.1161/HYPERTENSIONAHA.117.09539
7. Kim ESH, Olin JW, Froehlich JB, et al. Clinical manifestations of fibromuscular dysplasia vary by patient sex: a report of the United States registry for fibromuscular dysplasia. J Am Coll Cardiol 2013; 62(21):2026–2028. doi:10.1016/j.jacc.2013.07.038
8. Touzé E, Southerland AM, Boulanger M, et al. Fibromuscular dysplasia and its neurologic manifestations: a systematic review. JAMA Neurol 2019; 76(2):217–226. doi:10.1001/jamaneurol.2018.2848
9. Kadian-Dodov D, Gornik HL, Gu X, et al. Dissection and aneurysm in patients with fibromuscular dysplasia: findings from the US registry for FMD. J Am Coll Cardiol 2016; 68(2):176–185. doi:10.1016/j.jacc.2016.04.044FREE Full TextGoogle Scholar
10. Caps MT, Zierler RE, Polissar NL, et al. Risk of atrophy in kidneys with atherosclerotic renal artery stenosis. Kidney Int 1998; 53(3):735–742. doi:10.1046/j.1523-1755.1998.00805.
11. Guzman RP, Zierler RE, Isaacson JA, Bergelin RO, Strandness DE Jr.. Renal atrophy and arterial stenosis. A prospective study with duplex ultrasound. Hypertension 1994; 23(3):346–350. doi:10.1161/01.hyp.23.3.346Abstract/
12. Brinza EK, Gornik HL. Fibromuscular dysplasia: advances in under standing and management. Cleve Clin J Med 2016; 83(11 suppl 2):S45–S51. doi:10.3949/ccjm.83.s2.06
13. Olbricht CJ, Paul K, Prokop M, et al. Minimally invasive diagnosis of renal artery stenosis by spiral computed tomography angiography. Kidney Int 1995; 48(4):1332–1337. doi:10.1038/ki.1995.418
14. Postma CT, Joosten FB, Rosenbusch G, Thien T. Magnetic resonance angiography has a high reliability in the detection of renal artery stenosis. Am J Hypertens 1997; 10(9 Pt 1):957–963. doi:10.1016/s0895-7061(97)00157
15. Sabharwal R, Vladica P, Coleman P. Multidetector spiral CT renal angiography in the diagnosis of renal artery fibromuscular dysplasia. Eur J Radiol 2007; 61(3):520–527. doi:10.1016/j.ejrad.2006.10.005
16. Vasbinder GB, Nelemans PJ, Kessels AG, et al. Accuracy of computed tomographic angiography and magnetic resonance angiography for diagnosing renal artery stenosis. Ann Intern Med 2004; 141(9):674–682. doi:10.7326/0003-4819-141-9-200411020-00007
17. Textor SC. Pitfalls in imaging for renal artery stenosis. Ann Intern Med 2004; 141(9):730–731. doi:10.7326/0003-4819-141-9-200411020-00017
18. Olin JW, Piedmonte MR, Young JR, DeAnna S, Grubb M, Childs MB. The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann Intern Med 1995; 122(11):833–838. doi:10.7326/0003-4819-122-11-199506010-00004
19. Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy. Am J Hypertens 2010; 23(11):1159–1169. doi:10.1038/ajh.2010.174
20. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113(11):e463–e654. doi:10.1161/CIRCULATIONAHA.106.174526
21. Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med 2004; 350(18):1862–1871. doi:10.1056/NEJMra032393CrossRefPubMedGoogle Scholar
22. Kawasaki D, Fujii K, Fukunaga M, et al. Safety and efficacy of carbon dioxide and intravascular ultrasound-guided stenting for renal artery stenosis in patients with chronic renal insufficiency. Angiology 2015; 66(3):231–236. doi:10.1177/0003319714524297CrossRefPubMedGoogle Scholar
23. Raman G, Adam GP, Halladay CW, Langberg VN, Azodo IA, Balk EM. Comparative effectiveness of management strategies for renal artery stenosis: an updated systematic review. Ann Intern Med 2016; 165(9):635–649. doi:10.7326/M16-1053CrossRefPubMedGoogle Scholar
24. Beck AW, Nolan BW, De Martino R, et al. Predicting blood pressure response after renal artery stenting. J Vasc Surg 2010; 51(2):380–385. doi:10.1016/j.jvs.2009.08.088CrossRefPubMedGoogle Scholar
25. Tuttle KR, Chouinard RF, Webber JT, et al. Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis 1998; 32(4):611–622. doi:10.1016/s0272-6386(98)70025-3
26. Trinquart L, Mounier-Vehier C, Sapoval M, Gagnon N, Plouin PF. Efficacy of revascularization for renal artery stenosis caused by fibromuscular dysplasia: a systematic review and meta-analysis. Hypertension 2010; 56(3):525–532. doi:10.1161/HYPERTENSIONAHA.110.152918
27. Smit JV, Wierema TK, Kroon AA, de Leeuw PW. Blood pressure and renal function before and after percutaneous transluminal renal angioplasty in fibromuscular dysplasia: a cohort study. J Hypertens 2013; 31(6):1183–1188. doi:10.1097/HJH.0b013e32836044b2
28. Savard S, Steichen O, Azarine A, Azizi M, Jeunemaitre X, Plouin PF. Association between 2 angiographic subtypes of renal artery fibromuscular dysplasia and clinical characteristics. Circulation 2012; 126(25):3062–3069. doi:10.1161/CIRCULATIONAHA.112.117499
29. Olin JW. Is fibromuscular dysplasia a single disease? Circulation 2012; 126(25):2925–2927. doi:10.1161/CIRCULATIONAHA.112.149500
30. Lüscher TF, Keller HM, Imhof HG, et al. Fibromuscular hyperplasia: extension of the disease and therapeutic outcome. Results of the University Hospital Zurich cooperative study on fibromuscular hyperplasia. Nephron 1986; 44(suppl 1):109–114. doi:10.1159/000184059
31. Hughes JS, Dove HG, Gifford RW Jr., Feinstein AR. Duration of blood pressure elevation in accurately predicting surgical cure of renovascular hypertension. Am Heart J 1981; 101(4):408–413. doi:10.1016/0002-8703(81)90129-0CrossRefPubMedGoogle Scholar
32. Dworkin LD, Cooper CJ. Clinical practice. Renal-artery stenosis. N Engl J Med 2009; 361(20):1972–1978. doi:10.1056/NEJMcp0809200
33. Hricik DE, Dunn MJ. Angiotensin-converting enzyme inhibitor-induced renal failure: causes, consequences, and diagnostic uses. J Am Soc Nephrol 1990; 1(6):845–858. doi:10.1681/ASN.V16845Abstract/
34. Hollenberg NK. The treatment of renovascular hypertension: surgery, angioplasty, and medical therapy with converting-enzyme inhibitors. Am J Kidney Dis 1987; 10(1 suppl 1):52–60. pmid:3037890
Advertisement

One pediatric urologist’s quest to improve the status quo

First single-port renal vein transposition reduces recovery time and improves outcomes
Fully-automated process uses preop CT, baseline GFR to estimate post-nephrectomy renal function
Belzutifan superior to everolimus in phase 3 clinical trial
Management of high-risk RMSK in the pre-and current eras of neoadjuvant therapy
NIH-funded study explores novel MRI technique to stage cystic kidney disease
AI-generated model bests predictive abilities of human experts
Study highlights benefits of nephrologist-led urine sediment analysis