Unique model is an excellent genetic animal model for study of lethal diseases
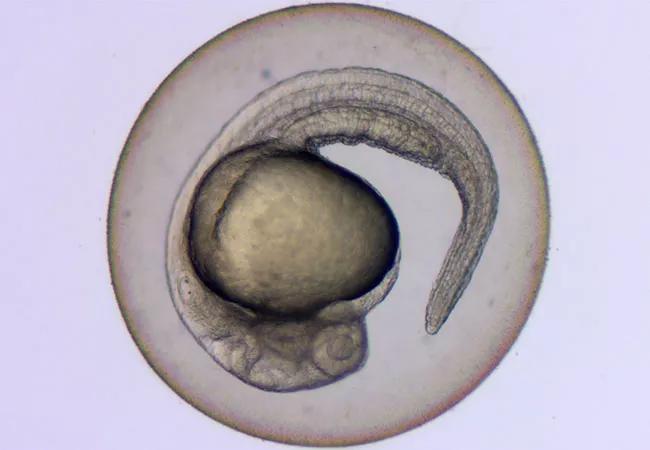
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b2691ec3-f72e-472d-b864-d2215d32a122/650x450-Ewing-sarcoma_jpg)
Zebrafish embryo
After having successfully created a zebrafish model to study Shwachman-Diamond syndrome (SDS), a rare inherited bone marrow failure syndrome that confers a high risk for developing myelodysplastic syndrome and acute myeloid leukemia, researchers at Cleveland Clinic are now turning their attention to other genetic disorders and cancers.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“My goal in coming to Cleveland Clinic was to develop a research program to study inherited neutropenias in children, and having an excellent animal model like zebrafish can really accelerate the discovery of treatments for these types of diseases,” says pediatric oncologist Seth J. Corey, MD, MPH, associate professor in the Department of Pediatric Hematology/Oncology and Cancer Biology at Cleveland Clinic Children’s. Dr. Corey was drawn to the institution by the opportunity to work with Jaroslaw Maciejewski, MD, PhD, FACP, Chair of Translational Hematology & Oncology Research, who is a world-renowned researcher for his work looking at the genetic basis for acquired, hybrid and inherited bone marrow failure syndromes such as aplastic anemia, paroxysmal nocturnal hemoglobinuria, myelodysplastic syndrome and large granular lymphocytic leukemia. Dr. Corey is also working with Usua Oyarbide, PhD, in the Department of Cancer Biology, who has generated a variety of zebrafish models, including ones for Ewing sarcoma (EWS) and SDS.
Zebrafish are tropical fish that are native to southeast Asia, and grow to approximately 2.5–4 cm in length. They are almost transparent, so their organs can be easily visualized. Zebrafish typically lay up to 100 eggs at a time at weekly intervals, which mature quickly in five days’ time. They are also less expensive to maintain than mice, which are the usual animal models employed, and take far less time to breed.
“Zebrafish are excellent organismal models to study for cancers and bone marrow failure syndromes,” says Dr. Oyarbide. She reports that the zebrafish aquatic facility consists of two rooms and one quarantine room at the Lerner Research Institute. In a room dedicated to her research, she has more than 400 tanks, where she is breeding mutant and transgenic lines for various projects.
Advertisement
Drs. Corey and Oyarbide are currently focusing on three neutropenias.
Shwachman-Diamond syndrome. “This disease is characterized by exocrine pancreatic insufficiency, neutropenia and skeletal abnormalities,” says Dr. Corey. It is an autosomal recessive disorder, and biallelic mutations in the SBDS ribosome maturation factor gene are found in 90% of children with the disease. Mice are not good animals for study of SDS as they die in the embryonic stage after being mutated. In an article recently published in JCI Insight, Drs. Oyarbide, Corey and others reported that SBDS is expressed at the 1-cell stage and during early embryonic stages in zebrafish. Using CRISPR/Cas9 editing techniques, they further reported that they were able to produce two stable SBDS mutant lines that can serve as phenotypes for human SDS.
Ewing sarcoma. EWS is one of the most common and lethal pediatric solid tumors, and new therapies are desperately needed. The vast majority of patients with EWS have a chromosomal translocation that encodes a EWSR1/FLI1 fusion protein, which spurs the development of tumors. Zebrafish with that fusion cancer protein are being made in the Corey-Oyarbide lab. In addition, EWS is notable for mutations in the TP53 gene, which mediates DNA damage repair, and the STAG2 gene, which is an important component of the cohesion complex associated with DNA replication. When either TP53 or STAG2 mutations are found with EWSR1/FLI1, the sarcoma is even more lethal. Drs. Oyarbide and Corey have received a grant from VeloSano Kids to study EWS in children and to use their fish to identify more effective therapies that their colleague Peter Anderson, MD, can evaluate in a clinical study
Advertisement
“The Ewing sarcoma project is coming along swimmingly, as we say,” reports Dr. Corey, and they have been able to utilize CRISPR to develop founder fish, which they are observing to make sure they have a stable line that is able to transmit the STAG 2 and TP53 mutations to the next generation.
Barth Syndrome. This is a rare X-linked mitochondrial disease that is due to a mutation in TAZ, a gene encoding the cardiolipin transacylase tafazzin. This inborn error of phospholipid metabolism leads to life-threatening cardiomyopathy, neutropenia, skeletal myopathy and growth delay. “When males are born with an absence of TAZ, they have less cardiolipin, which is a phospholipid found in the inner leaflet of the mitochondrion and required for the activity of the mitochondrial respiratory chain,” explains Dr. Corey.
The team was able to create two different zebrafish mutant lines using CRISPR/Cas9 targeting for the TAZ Exon 1. “We were surprised to find that two of our mutant lines can survive for six months, and we are now targeting Exons 3 and 4,” reports Dr. Corey. In collaboration with biochemist Yana Sandlers, PhD, of Cleveland State University, they are currently looking to do a detailed analysis of the phenotypes of zebrafish mutants Dr. Oyarbide has created, with a particular emphasis on cardiac mitochondrial morphology. They also want to determine the stress responses involved in the absence of tafazzin, with the ultimate goal of studying the pathogenesis of Barth syndrome, as well as identifying small molecule drugs, mitochondrial repair approaches and dietary modifications that could be used to rescue human mutations.
Advertisement
“Given our ongoing success with the zebrafish model, I hope pediatric cardiologists and others will be interested in collaborating on our research,” says Dr. Corey. He adds that he hopes to establish a robust center for the study of bone marrow failure using zebrafish models, so that Cleveland Clinic becomes a major referral center for patients with acquired and inherited neutropenias.
Advertisement
Advertisement
Goal-of-care discussions drive earlier hospice access
Clinical trials and de-escalation strategies
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting