One approved non-hormonal therapy and another on the horizon reduce vasomotor symptoms

Up to 80% of menopausal women experience hot flashes and night sweats, and 20% have severe vasomotor symptoms (VMS). Until recently, there were few options for relief aside from hormonal therapy. But in May 2023, the FDA-approved non-hormonal fezolinetant for VMS.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Hormonal therapy remains the gold standard, but we can’t use it for everyone, and not every candidate is willing to take it,” says Holly Thacker, MD, FACP, director of Cleveland Clinic’s Center for Specialized Women’s Health. “It’s very important that we have options to offer patients.”
Fezolinetant, which was developed by Astellas Pharma and will be marketed under the brand name Veozah, blocks the neurokinin 3 receptor, which contributes to the brain’s regulation of body temperature.
“When someone starts having either fluctuating levels of estrogen or absolute loss of estrogen, the KNDy neurons [kisspeptin, neurokinin B and dynorphin] in the hypothalamus get larger and that creates a cascade of temperature instability,” says Dr. Thacker. “By acting as an antagonist on the NK3 receptor, fezolinetant can help reduce vasomotor symptoms.”
Veozah is a 45 mg pill taken once daily. It’s the first FDA-approved drug in a family of NK3 receptor antagonists. Bayer is developing elinzanetant, an NK1 and NK3 receptor antagonist that is currently in phase III clinical trials. In the Skylight 2 phase III clinical study of fezolinetant, postmenopausal women aged 40 to 65 who experienced at least seven moderate to severe hot flashes daily were given either a placebo, a 30 mg dose of the drug, or 45 mg dose of the drug for 12 weeks. All participants were then transitioned to active fezolinetant for 52 weeks. Fezolinetant reduced the daily mean for hot flashes from 11 to five in women taking the 30 mg dose, and from 12 to four in women taking 45 mg. (More details about the study are available in a podcast presented on the Speaking of Women’s Health™ digital platform.)
Advertisement
Development of non-hormonal therapy for VMS was serendipitous.
“We stumbled upon non-hormonal options that alleviate menopausal symptoms while treating people for other problems, who then told their doctors, ‘Hey, by the way, I’m not having hot flashes as frequently,’” says Dr. Thacker.
For example, selective serotonin reuptake inhibitors (SSRIs), such as paroxetine, seem to reset the brain thermostat and reduce VMS. Paroxetine (brand name Brisdelle, Paxil, Paxil CR and Pexeva) has been FDA-approved to treat VMS. However, it can’t be used in patients with breast cancer who are taking tamoxifen.
There are similar counterindications for hormone replacement therapy (HRT), including women being treated for estrogen-positive breast cancer or endometrial cancer, people with blood clots or who are at risk for them, and women of advanced age with a history of stroke or cardiovascular risks. New non-hormonal options present a good alternative for them, as well as for women who decline HRT.
“Unfortunately, the urban myth overstating the dangers of hormones scares many women away from HRT,” says Dr. Thacker. “For the vast majority of women – especially if they start hormonal therapy under age 65 and within 10 years of menopause – there is significant benefit not only in symptom control, but also in long-term reduction in death rates and several significant diseases.”
While HRT remains the optimal treatment for many women for menopausal symptoms, Dr. Thacker recognizes the importance of non-hormonal options.
Advertisement
“The drug is metabolized in the liver and there can be some drug-drug interactions, so the FDA requires liver function tests at the 3-, 6- and 9-month mark,” she says.
“Normally, we see our patients three months after a menopause evaluation, whether we start hormonal or non-hormonal therapy,” she says. “We will do the same with fezolinetant, and I will certainly follow the recommendations and order future labs.”
Dr. Thacker offers an additional piece of advice to women’s health practitioners.
“Physicians, nurse practitioners and physician assistants from many backgrounds will feel comfortable prescribing 45 mg of Veozah per day,” she says. “But that does not absolve you from knowing the bone density, cardiovascular and genitourinary status of your patients. I recommend you do a comprehensive midlife anti-aging assessment to ensure you are offering the most appropriate therapy.”
She is excited by the development and approval of non-hormonal options and looks forward to more menopausal therapies are on the horizon.
“Going through menopause is universally experienced by adult women who live long enough,” says Dr. Thacker. “We have been desperately in need of therapeutic options, and the fact that we now have a first-in-class new medication is very helpful.”
Advertisement
Advertisement

VMS in menopausal women can lead to adverse health outcomes

Approximately 500 million people globally are experiencing 'period poverty'

Mode of delivery does not affect patient satisfaction

A case-based discussion of efficacy, eligibility and use

ACC committee underscores need to properly weigh benefits in risk-benefit calculations
Treatment being offered in cases where medical and hormonal management was not successful
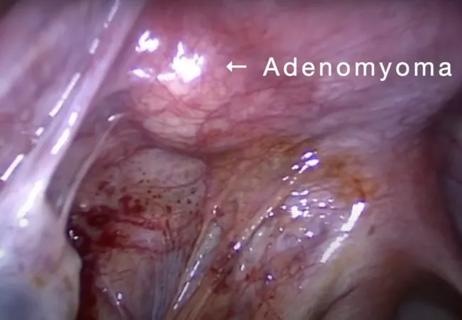
Laparoscopic surgery provides relief for teenage patient with adenomyoma and endometriosis
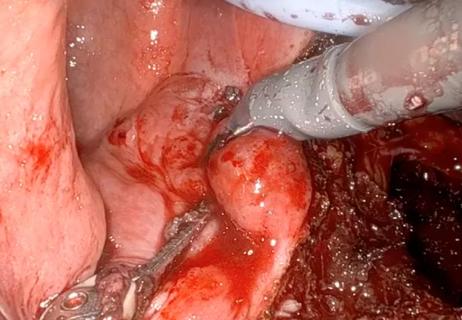
A large nodule had strangulated the patient’s ureter and invaded the vagina, bladder and rectum