What's driving tumor progression is the key, says Nima Sharifi, MD

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/15f4743b-14b9-46d4-a7e1-b80266992d59/650x450-Sharifi-2015-in-lab-1_jpg)
650×450-Sharifi-2015-in-lab-1
In the last decade, the Food and Drug Administration has approved a variety of new drugs for the treatment of advanced castration-resistant and castration-sensitive prostate cancer, including cabazitaxel, enzalutamide, abiraterone and apalutamide.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The current design of clinical trials that determine whether new agents such as these are approved, or existing ones are expanded for new indications, is based on whether or not patients have metastatic disease, and on previous treatments.
That paradigm must change if therapies for advanced prostate cancer — particularly those with curative intent for oligometastatic disease — are to progress, contends Cleveland Clinic oncologist and cancer researcher Nima Sharifi, MD. “You’ve got to take a much more rational approach, in my opinion,” he says. “You need to understand what’s driving tumor progression, what treatment will add benefit, and for which patients.”
Dr. Sharifi is Director of Cleveland Clinic’s Center for Genitourinary Malignancies Research, Co-Director of the Center of Excellence in Prostate Cancer Research, and Director of the Genitourinary Program of the Cleveland-wide Case Comprehensive Cancer Center. His landmark research has focused on prostate oncogenesis, the biology of androgen metabolism and mechanisms of androgen deprivation therapy resistance in advanced prostate cancer.
At the American Urological Association’s 2019 annual meeting, Dr. Sharifi’s presentation outlined a strategy to “move the needle” on treatment. He elaborated in this interview with Consult QD.
Dr. Sharifi: Androgen deprivation therapy has been the longstanding backbone of care for men with metastatic disease. In the past few years, there have been tremendous advances in the development of new chemotherapy and hormone therapy agents. We see survival advantages for many of these. But the way we’ve structured these clinical trials, and the way the FDA approvals have gone forward, has not been based on the intrinsic biology of the clinical disease, meaning what’s driving tumor progression. In contrast, it’s all based on what the patient or the tumor has or has not been treated with, and whether or not the patient has metastatic disease.
Advertisement
Dr. Sharifi: One would think your approach should be based on the biology of the disease and not the limitations of the treatments we have in clinic. At present, the registration trials and FDA approvals are based on whether a patient has been treated with an androgen receptor antagonist or chemotherapy and has metastatic disease. If a patient has been treated with an agent and their disease has progressed, unless they’ve come off treatment due to adverse effects or side effects, the general premise is that it’s because the tumor has become resistant to that treatment. There’s no biologic distinction made among patients in any of these trials. We don’t take into account biologic drivers that vary from tumor to tumor and patient to patient. I think it makes more sense to reconsider this once we have a foundational knowledge of what drives the tumor. If you understand the inner workings of the tumor cell — what it responds to, what it’s addicted to — then you may be able to come up with a system that makes more sense than something that is largely empiric.
Dr. Sharifi: Yes. Cancer is a disease of genetics. In regard to the patient, there are germline genetics. Physiology is inherited through the germline and clearly regulates clinical outcomes in prostate cancer, under conditions of hormone therapy treatment. And a tumor is the result of somatic genetic changes. You really need to understand the genetic context of the host — the patient in which the tumor is growing — and the genetic context of the tumor, what it’s addicted to. That’s the essential framework upon which to pursue clinical trials, especially those with curative intent. You can boil it down to two questions regarding mechanisms of resistance: Does the tumor need androgen? And does the tumor get androgen?
Advertisement
Dr. Sharifi: In prostate cancer, the androgen receptor is the major driver. In normal physiology, that’s driven by gonadal testosterone, because that’s the most abundant and potent androgen we have, and that dictates tumor progression. So the standard of care had been to get rid of these androgens. But we also know that when you get rid of the gonadal source, you have a huge pool of androgen precursors in circulation that can also feed the tumor. They have to be converted to testosterone or DHT (5α-dihydrotestosterone).
So getting at the question of does a tumor need androgen, there are a variety of studies that address whether a tumor expresses androgen receptor and whether it is dependent on androgens. Gene expression signatures have been developed, using the somatic context of the tumor itself. Other studies have shown that when you lose certain tumor suppressor genes, tumors are less and less addicted to androgens and are basically autonomous; they grow by themselves and don’t require external signals.
Whether the tumor gets androgen is a different question. Adrenal DHEA (dehydroepiandrosterone, an androgen precursor) can be sequentially converted by enzymes to testosterone or DHT. The enzyme 3βHSD1 is required for any pathway to get from extragonadal androgen precursors to the most potent DHT. The gene that encodes for the enzyme is HSD3B1. There’s a very common germline variant where the protein coding change differs substantially by race. There’s what I call an adrenal-restrictive genotype and an adrenal-permissive genotype. The adrenal-restrictive genotype encodes for a protein that’s rapidly degraded, so there is very slow conversion from DHEA to DHT, leading to low levels of DHT synthesis. In contrast, the adrenal-permissive genotype encodes for a stable protein, with rapid conversion from DHEA to DHT and high levels of DHT in the tumor tissue. The adrenal-permissive genotype opens the floodgates to DHT, allowing for clinical progression to castration-resistant disease.
Advertisement
So the genotype really confers clinical outcomes. Seventy percent of Spanish and Italian men harbor at least one copy of the adrenal-permissive allele, while the percentage is much lower, about 9%, among men from certain parts of Nigeria and South America. Everyone else is in between. You can imagine that, with different drivers of progression in different hosts, these men require different therapies.
Dr. Sharifi: Ideally, you would want to know about tumor-intrinsic androgen receptor dependency and about patient-dependent androgen metabolism. This is one way of potentially moving the needle: You parse things out between tumors that are androgen receptor-dependent and those that are androgen receptor-indifferent, and patients with the adrenal-permissive genotype or the adrenal-restrictive genotype.
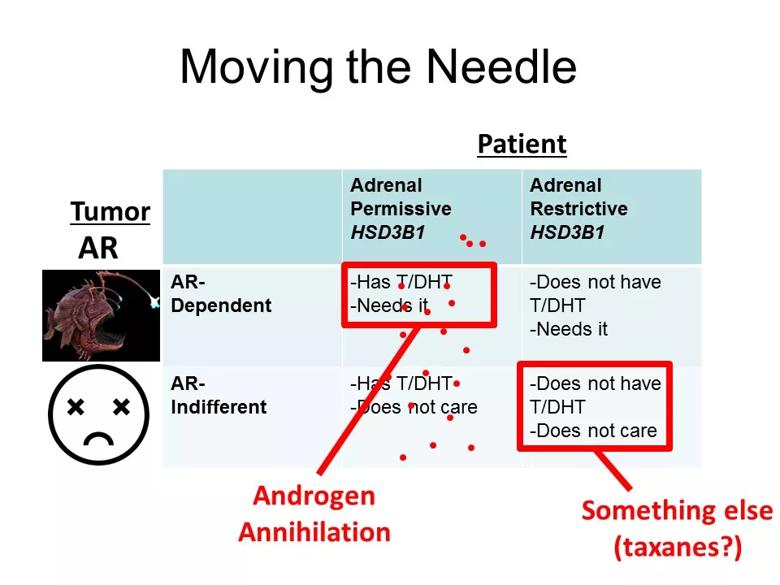
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2a4722e4-50a0-4f1a-b62f-d027fec86053/AUA-SBUR-SUO-5-4-2019_jpg)
This slide from Dr. Sharifi’s American Urological Association presentation illustrates the interaction of tumor-intrinsic androgen receptor dependency and patient-dependent androgen metabolism.
For example, patients with adrenal-permissive genotype and an androgen receptor-dependent tumor probably would respond better to androgen annihilation. In contrast, patients that are adrenal-restrictive and whose tumor is androgen receptor-indifferent may benefit to a greater extent from something else — maybe taxanes or an experimental agent. That’s the basic proposed framework.
Dr. Sharifi: It’s up to a number of stakeholders: Clinical investigators. The pharmaceutical industry, because they support the trials. Patients. Maybe I’m wrong for some reason and we need to incorporate other aspects of biology. Or maybe there are other regulatory or structural limitations to doing this that need to be flushed out. The reality is that it requires a dialogue, and moving toward a different framework.
Advertisement
Advertisement
First full characterization of kidney microbiome unlocks potential to prevent kidney stones
Researchers identify potential path to retaining chemo sensitivity
Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time
Investigators are developing a deep learning model to predict health outcomes in ICUs.
Preclinical work promises large-scale data with minimal bias to inform development of clinical tests
Cleveland Clinic researchers pursue answers on basic science and clinical fronts
Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches
Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology