New pathways to consider in the treatment of SSc-ILD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Interstitial lung disease (ILD) is a leading cause of morbidity and mortality in systemic sclerosis (scleroderma, SSc). It is a consequence of the interplay of disordered immunologic, fibrotic and vascular pathways that culminates in nonreversible scarring of the lungs (Figure). Although the traditional approach to the treatment of SSc-ILD has focused on immunosuppression, there is a rationale for targeting the other two pathways.
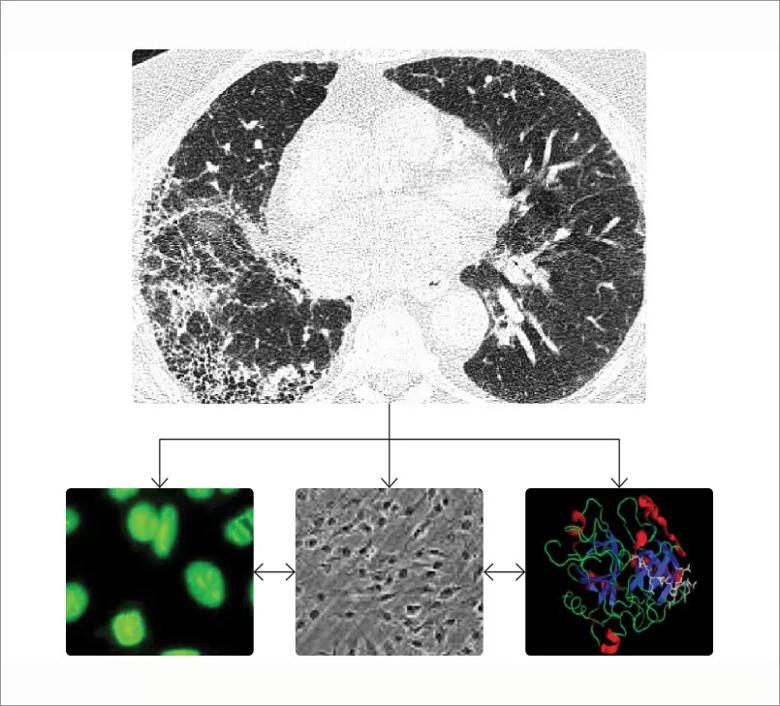
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2cf2b6ae-85cb-498f-b467-0fa0ac30cf12/805x-SSc-ILD_jpg)
Figure. SSc-ILD is a consequence of the interplay of disordered immunologic, fibrotic and vascular pathways. The figure shows a SSc-ILD on a chest computed tomography, the Scl-70 auto-antibody, lung fibroblasts and the thrombin molecule.
The first Scleroderma Lung Study (SLS I)1 studied oral cyclophosphamide versus placebo. In this study, there was a significant but modest improvement in forced vital capacity (FVC), dyspnea, skin thickening and health-related quality of life. Unfortunately, these effects were no longer apparent one year after discontinuation of therapy,2 and the toxicity of cyclophosphamide precludes its long-term use. These findings prompted the SLS II,3 which compared two years of mycophenolate mofetil (MMF) to one year of oral cyclophosphamide followed by one year of placebo. In this study, both drugs resulted in significant improvements in FVC, and MMF was associated with less toxicity. The hypothesis that two years of MMF would have greater efficacy than one year of cyclophosphamide at 24 months was not confirmed. Nevertheless, MMF has become the standard of care for SSc-ILD in the United States. Promising clinical trials for SSc-ILD Tocilizumab, targeting IL-6, looks promising for SSc-ILD based on subgroup analysis of the focuSSced (A Study of the Efficacy and Safety of Tocilizumab in Participants With Systemic Sclerosis) and faSScinate (Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomized controlled trial) clinical trials, which were designed to examine the effects of tocilizumab on skin fibrosis.4,5 Finally, autologous stem cell transplantation is emerging as a novel treatment option for refractory disease based on the outcomes of three randomized controlled trials (ASSIST, SCOT and ASTIS), which included patients with SSc-ILD, that showed improved event-free survival and modest improvement in pulmonary function.6-8
Advertisement
Based on the observation that the anti-fibrotics nintedanib and pirfenidone slowed the rate of FVC decline in idiopathic pulmonary fibrosis, these drugs have also been studied in SSc-ILD. In the phase 2 LOTUSS trial, pirfenidone was found to be safe and well tolerated in patients with SSc-ILD.9 With regard to efficacy, we await the results from SLS III, which compares initial combination pirfenidone plus mycophenolate versus placebo plus mycophenolate over 18 months in patients with newly diagnosed SSc-ILD (clinicaltrials.gov: NCT03221257). The SENSCIS study9 was a multinational, phase 3 randomized, double-blind, placebo-controlled trial that investigated the efficacy and safety of nintedanib in 580 patients with SSc-ILD. Patients were randomly assigned to receive 150 mg of nintedanib twice daily or placebo. Approximately 50% of patients were on stable background mycophenolate. There was a significant reduction in the adjusted rate of change in FVC (-52.4 mL/year in the nintedanib group versus -93.3 mL/year in the placebo group [difference, -41.0 mL/year; P = 0.04]). Based on these data, nintedanib became the first drug approved by the Food and Drug Administration for SSc-ILD on Sept. 6, 2019, changing the treatment paradigm for SSc-ILD.
Substantial evidence supports a “vascular hypothesis” for the pathogenesis of SSc-associated ILD. Not only is thrombin elevated in the bronchoalveolar lavage fluid of patients with SSc-ILD, but thrombin can induce the myofibroblast phenotype. Therefore, targeting thrombin with a direct thrombin inhibitor could prove to be a novel and effective treatment strategy based on preclinical data. As a first step toward designing a clinical trial to test the efficacy of thrombin inhibition in SSc-ILD, a single-center, open-label treatment trial with the direct thrombin inhibitor dabigatran was performed in patients with SSc-ILD.10 Dabigatran appeared to be safe and well tolerated. In addition, thrombin activity in bronchoalveolar lavage fluid decreased or remained stable in 92.8% of the subjects.
Advertisement
Max Hirsch, MD, the father of modern rheumatology, once said that “no drug has truly failed until it has been tried in scleroderma.” As a result of a better understanding of the pathobiology of SSc-ILD and its multiple pathways, the future of SSc-ILD is much brighter.
1. Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655-2666.
2. Tashkin DP, Elashoff R, Clements PJ, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176(10):1026-1034.
3. Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med.2016;4(9):708-719.
4. Khanna D, Denton CP, Lin CJF, et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomized controlled trial (faSScinate). Ann Rheum Dis.2018;77(2):212-220.
5. Khanna D, Denton CP, Jahreis A, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387(10038):2630-2640.
6. Burt RK, Shah SJ, Dill K, et al. Autologous nonmyeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomized phase 2 trial. Lancet. 2011;378(9790):498-506.
7. van Lear JM, Farge D, Sont JK, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014;311(24):2490-2498.
8. Sullivan KM, Goldmuntz EA, Keyes-Elstein L, et al. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N Engl J Med. 2018;378(1):35-47.
9. Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis associated interstitial lung disease. N Engl J Med. 2019;380(26)2518-2528.
10. Silver RM, Wilson DA, Akter T, Huggins JT, Kajdasz K, Highland KB, Nietert PJ, Bogatkevich GS. Safety and Tolerability of Thrombin Inhibition in Scleroderma-Associated Interstitial Lung Disease. ACR Open Rheumatol. 2019;1(7):403-411.
Advertisement
Dr. Highland is a pulmonologist/critical care physician and a rheumatologist. She is Director of the Rheumatic Lung Disease Program. She was an investigator in the SLS II and dabigatran studies and the coglobal lead of the SENSCIS trial.
Advertisement
Advertisement
Takeaways from the most recent annual meeting centered around clinical advances, AI integration and professional development
Recent breakthroughs have brought attention to a previously overlooked condition
A review of treatment options for patients who may not qualify for surgery
Looking at the real-world impact and the future pipeline of targeted therapies
The progressive training program aims to help clinicians improve patient care
New breakthroughs are shaping the future of COPD management and offering hope for challenging cases
Exploring the impact of chronic cough from daily life to innovative medical solutions
How Cleveland Clinic transformed a single ultrasound machine into a cutting-edge, hospital-wide POCUS program