Evidence-based, internally validated method for risk evaluation

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/edc7ec8b-52e5-4e08-b79a-d1b3e8cdb7b7/HEN-1872897_03-26-20_011_RN-jpg)
NSAID
A group of clinicians from Cleveland Clinic and Harvard University have developed a risk score to predict which patients might be susceptible to toxicities from chronic nonsteroidal, anti-inflammatory drug (NSAID) use. This post hoc analysis of the Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen or Naproxen (PRECISION) trial offers an internally validated method for risk evaluation based on data from thousands of patients taking ibuprofen, naproxen or celecoxib chronically.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Chronic NSAID use can result in a wide range of toxicities, including gastrointestinal bleeding, acute and chronic kidney injury, heart failure and hypertension. In addition, patients chronically using NSAIDs often are of advanced age and face multiple comorbidities. Yet there is no biomarker for determining which patients will experience these adverse side effects.
“As physicians, we monitor patients who are using NSAIDs long-term, but sometimes these side effects come without warning,” says Elaine Husni, MD, MPH, Vice Chair of the Department of Rheumatologic and Immunologic Diseases at Cleveland Clinic.
This study capitalized on the wealth of data from PRECISION, a double-blind, randomized, controlled, multicenter study that tested whether the COX-2 inhibitor celecoxib was noninferior to naproxen and ibuprofen with respect to cardiovascular toxicity.
“We leveraged data from the large clinical trial to determine the major NSAID toxicities and risk factors so we can be better detectives about who is most likely to get these side effects,” says Dr. Husni.
The PRECISION trial found that celecoxib was noninferior to ibuprofen or naproxen with respect to cardiovascular safety. A secondary analysis in which researchers developed a composite safety endpoint for major NSAID toxicity that included cardiovascular, gastrointestinal, renal and all-cause mortality was published in Arthritis & Rheumatology.
In the current analysis, researchers used TRIPOD recommendations for developing a prediction model. Patients recruited during the first four years (N = 15,196) of enrollment were used to derive a risk score, and patients enrolled in the last five years (N = 8,757) were used for validation. The team found the following risk factors for developing NSAID toxic side effects:
Advertisement
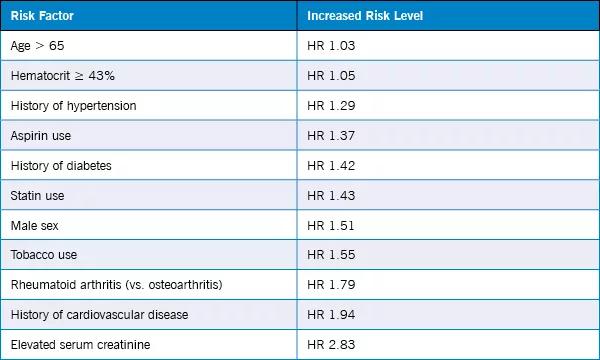
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6758f465-c1c0-47e5-a77e-f01af67ed20c/19-RHE-1010-HusniNSAIDriskScoreTable-1-600x300_jpg)
Daniel Solomon, MD, of Brigham and Women’s Hospital; Dr. Husni and their coauthors found that:
“We created three categories: very low risk, less than 1%; moderately at risk, 1 to 4%; and very high risk, over 4%,” says Dr. Husni. “If a patient is in the very high risk category, you may consider alternatives to NSAIDs or you may start them on a very low dose and monitor them more closely.”
The risk factors that make up the toxicity score are easy to obtain and offer physicians and clinicians an evidence-based way to evaluate a patient’s risk, says Dr. Husni. She adds that although potential toxicity from chronic NSAID use is a concern, it does not rise to the level of concern with opioid use.
“Pain represents the most common reason for physician visits, and healthcare providers in the United States write more than 100 million NSAID prescriptions annually,” she says. “Although potential NSAID toxicities are numerous, the recent attention on the opioid abuse epidemic and its consequences still will support the use of NSAIDs. Studies like this one will help to optimize chronic NSAID use.”
Advertisement
Advertisement
Researching the biological basis for why treatment is or is not effective
Scribing system helps create more face-to-face interactions
A conversation with Leonard Calabrese, DO
The case for continued vigilance, counseling and antivirals
High fevers, diffuse rashes pointed to an unexpected diagnosis
No-cost learning and CME credit are part of this webcast series
Summit broadens understanding of new therapies and disease management
Program empowers users with PsA to take charge of their mental well being