A conversation with Feixiong Cheng, PhD, about his wide-ranging research initiatives

Although more than a century has passed since Alzheimer’s disease (AD) was first characterized, prevention and effective treatments have remained elusive.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
In an effort to spur progress against this devastating, immensely complex condition, Cleveland Clinic researcher Feixiong Cheng, PhD, has devised a multipronged research strategy that integrates advanced computational and experimental approaches.
Dr. Cheng (shown above), who directs the Lerner Research Institute’s Alzheimer’s Network Medicine Laboratory, began his career at Cleveland Clinic studying cardio-oncology but shifted his primary focus to AD and related dementias (ADRD) for compelling personal reasons. He collaborates with investigators in multiple specialties across the institution and around the world, drawing on multi-omics, artificial intelligence, network medicine, systems biology assays and other advanced methodologies (e.g., patient stem cell-derived brain organoids) to enhance understanding of ADRD on various fronts.
In 2023 alone, his team’s research plans have attracted more than $16 million in grant support from the National Institutes of Health (NIH) and the Alzheimer’s Association (see sidebar).
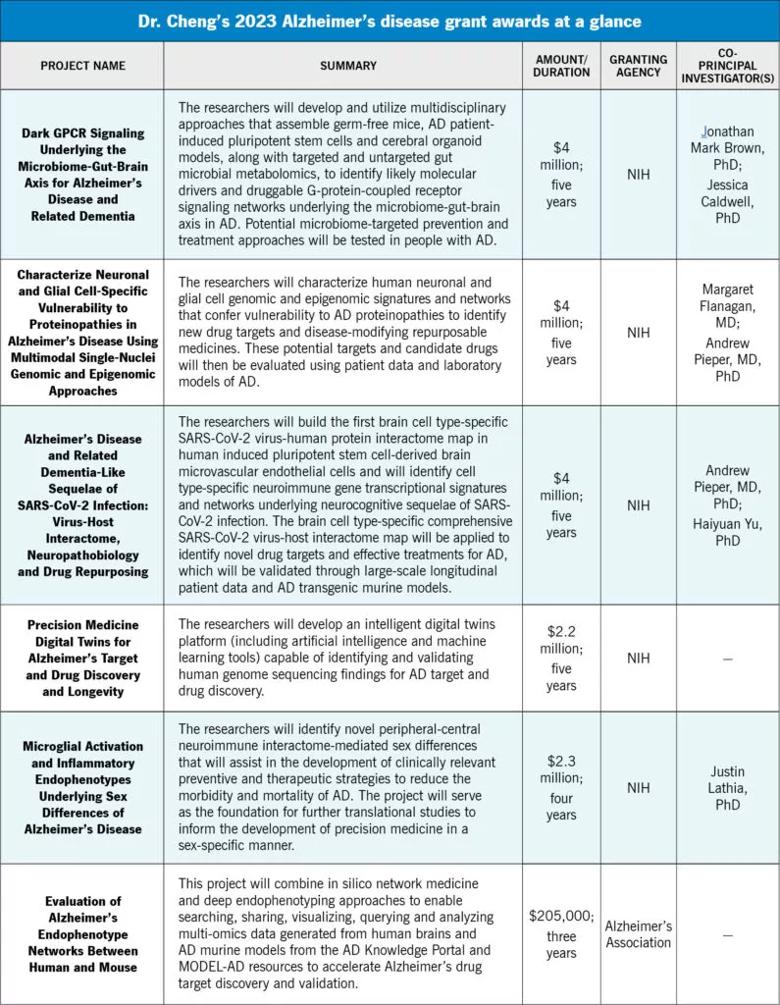
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a6b5f505-f879-4aac-8342-7b61835a8903/23-NEU-4216627-CQD-Inset-800x1030-v2-795x1024_jpg)
In a wide-ranging conversation with Consult QD, Dr. Cheng discusses the challenges AD poses, the need for a multidisciplinary precision medicine approach, the rationale for his various tactics and the impact he hopes his research will make. The interview has been edited for brevity.
Your past research has dealt with a variety of diseases and conditions, from atrial fibrillation and arteriovenous malformations to COVID-19 and various cancers. Why did you decide to focus on AD and related dementia?
Advertisement
I think the main reason, my personal reason, is that my grandfather died of dementia 15 years ago when I was an undergraduate student. I studied heart disease and I came to Cleveland Clinic to do cardio-oncology research. But I also saw the opportunity to work on this terrible disease based on network systems biology technologies I was trained in at Harvard Medical School and Vanderbilt University Medical Center, and I just immediately jumped at that. I still do cardio-oncology research, but my main focus is on Alzheimer’s disease and related dementia.
AD was identified more than a century ago, yet there is still no curative or preventive treatment. Why has it been so difficult to unravel?
It’s a highly complex disease. We use genetics and multi-omics, but most of the data we gather is from specimens from patients who have donated their brains for study after they die, at the end of their disease. We try to identify drug targets from these specimens and then test candidate drugs in patients with earlier-stage Alzheimer’s. That’s a challenging gap. Another challenge, not just for Alzheimer’s but for all brain disorders, is the blood-brain barrier “brain evolution.” Our brains have evolved a very smart protection system. A potential therapeutic agent may have good pharmacokinetics in the peripheral blood system, but that molecule cannot get to the brain.
Is some of the challenge due to Alzheimer’s multifactorial nature, involving neuroinflammation, dysfunction in autophagy, protein misfolding and more?
Advertisement
Exactly. A key concept in my research is approaching Alzheimer’s disease as multiple intermediate endophenotypes — amyloidosis, tauopathy, neuroinflammation, and mitochondrial, vascular and lysosomal dysfunction. The multiple endophenotype hypothesis is a biological definition of the disease, not a clinical definition. We can use an imaging approach, a biomarker approach, a genetic approach to define Alzheimer’s disease biology or biological change at an earlier stage than is possible clinically.
You’ve said that it’s still a great challenge in Alzheimer’s to translate genetics and multi-omics findings into disease pathobiology, which makes it hard to develop new therapeutics. Why is that?
Most of the genetic approaches we use today involve genome-wide association or whole-genome studies. The problem is that 98% of the human genome consists of noncoding regions. We can identify genetic loci that are associated with risk or are protective for Alzheimer’s, but because they’re in noncoding regions, we can’t identify druggable targets. Also, Alzheimer’s is not caused by genetics alone, but is an interaction between genetic and environmental factors. Altogether, it is challenging to translate the genetic findings and even multi-omics findings into a therapeutic discovery.
In the last few months, you’ve received more than $16 million in grant awards to support your AD research. To what do you attribute that success?
I think our success is based on some fundamental systems biology technology, AI technology and genomic medicine technology that we developed at Cleveland Clinic in the past four or five years. In addition, the outstanding research environment and clinical research resources here also offer first-line patient data to make it possible to test various translational therapeutic hypotheses in ADRD. That has provided us with some striking preliminary data to make a convincing case that we have a chance to successfully translate these findings into therapeutic discoveries and patient care.
Advertisement
A common thread in the research supported by these grants is your use of systems biology and network medicine to better understand AD and identify new therapeutic targets. Why are those approaches essential, and what does it take to assemble a team with the capabilities to do this advanced research?
I want to thank all my previous mentors for providing training in systems biology and network medicine, which really helped me launch my career and use this approach to study Alzheimer’s disease. Because of the disease’s complexity, we cannot take a reductionist single-gene or single-mutation approach. We really needed a multifactorial effort, considering genetics, epigenetics, multi-omics and environmental factors together to build global knowledge, like a brain protein-protein interactome map, to understand the complexity of this disease. Then we can develop strategies and work on therapeutic targets. We really have a multidisciplinary team at Cleveland Clinic and beyond. For example, I work with physicians like James Leverenz, MD, Director of the Cleveland Alzheimer’s Disease Research Center. He can provide unique patient-based specimens and multi-omics data to allow us to do this kind of research. I work with Jeffrey Cummings, MD (founding director of Cleveland Clinic’s Lou Ruvo Center for Brain Health and director of the Center for Neurodegeneration and Translational Neuroscience), who is a leading Alzheimer’s clinical researcher and has given me a lot of help and insights. I also work with Andrew Pieper, MD, PhD, from Case Western Reserve University, who has expertise in preclinical models of Alzheimer’s. We have really great collaborators. Our team also operates both dry (computational) and wet labs. The dry lab does computational work, such as bioinformatics and multi-omics data analysis. We have data science people and expertise in machine learning and AI. The wet lab uses functional models, like patient stem cell-derived neuron and brain organoid models, to help us further validate our big data findings. We also use transgenic murine models to help make sure the molecules we identify can pass the blood-brain barrier, and to determine their safety profile.
Advertisement
You’re particularly interested in using human induced pluripotent stem cells and organoid models of the brain in your AD research, for modeling of disease pathophysiology and for drug discovery.
Yes. A challenge in Alzheimer’s drug discovery is translating findings from preclinical models into effective human treatments. We really need to generate findings from patient data or patient samples. This is why we work on Alzheimer’s patient-derived neurological models using induced pluripotent stem cells and cerebral organoids. With induced pluripotent stem cells, we can derive neurons, microglia, astrocytes — key cell types that are relevant to disease pathology. We can use these models to help us to look at drugs’ mechanisms of action. But single-cell models cannot simulate our complex brain because they lack cell-cell interactions. The brain organoid model, with neurons, astrocytes and microglia together in a 3D co-culture, is closer to human brain physiology. This is something we are working on now and have had some preliminary success.
Another common thread in your Alzheimer’s research is the use of AI and big data.
I love AI. The AI approach really helps us integrate multiple layers of data — like gene expression, protein expression, even patient clinical data — to build a comprehensive biological model and make highly accurate predictions. With the traditional statistical approach, we can only test one hypothesis. But with AI and deep learning, we can put all kinds of hypotheses together, or even test interactions between different hypotheses, to provide the best predictive model for the questions we seek to answer.
What kinds of big data resources do you use?
We work with IBM to access their MarketScan Medicare Claims database, which includes data on millions of individuals in the U.S. healthcare system. Mining these data helps us identify candidate drugs that potentially can be used to treat Alzheimer’s. For example, our analysis of the database showed that users of sildenafil had a significantly reduced risk for Alzheimer’s compared with sildenafil nonusers or users of other comparator drugs. We also work with our colleagues in the Cleveland Alzheimer’s Disease Research Center and the NIH/NIA-funded Alzheimer’s Disease Sequencing Project to access patients’ clinical, genetic, blood biomarker and phenotyping data. And now we can leverage large-scale data from the hundreds of thousands of specimens in Cleveland Clinic’s BioRepository collected from across our healthcare system, which is a huge opportunity for us.
One of your new research projects builds on your previous work that identified commonalities between the underlying mechanisms of AD and those of dementia-like cognitive impairment in COVID-19. Explain the connections.
During the pandemic, we had a few high-profile papers where we used the network-based prediction model approach we had built for Alzheimer’s to identify targets for drug repurposing in COVID-19. In 2021 we published about using network medicine to find significant mechanistic overlap between Alzheimer’s and COVID-19 in two areas. One is neuroinflammation, which may increase the risk for cognitive dysfunction in COVID-19 patients, particularly those who are hospitalized for severe infection. The other is brain microvascular injury, which also may increase the risk for cognitive dysfunction. Neuroinflammation and brain microvascular injury also are two major risk factors for Alzheimer’s disease and related dementia. We will further explore these apparent linkages in our ongoing research. Hopefully we can find biomarkers that identify patients at highest risk of neurological complications from COVID-19, as well as therapeutic targets that could lead to effective treatments for Alzheimer’s disease.
Are you concerned that there might be an increase in Alzheimer’s-like dementia because of the COVID pandemic?
Based on the epidemiology data published so far and on some of our preliminary data, I think so. Why? Because COVID-19 really targeted the aging population, in particular those in nursing homes. What we have learned so far is that those older individuals who have severe disease have neuroinflammation and experience impaired cognitive function for months or even years after they have COVID-19. I think the pandemic may increase the healthcare burden from many conditions, including dementia. Hopefully we can figure that out in the future.
If you were able to determine why COVID-19 predisposes some people to Alzheimer’s-like dementia, would that be a translatable finding? Could you use that knowledge to develop a therapy for Alzheimer’s?
That is our hypothesis. We are looking at linkages between COVID-19 and Alzheimer’s not just to find ways to treat pandemic-related dementia, but to find targets to treat Alzheimer’s as well. There are multiple studies that show evidence that viral and bacterial infections are associated with an increased risk for Alzheimer’s disease or other neurodegenerative diseases. One virus, HSV-1, appears to significantly increase the risk for Alzheimer’s, and we are planning research to look at this association as well.
You’re also planning to explore the gut-brain connection and its relationship to Alzheimer’s, correct?
Yes. This project came from a very simple idea. We got it from listening to a fabulous talk by Stanley Hazen, MD, PhD, about gut metabolites and heart disease. Our lab focuses on drug repositioning studies — identifying FDA-approved drugs that have potential to treat Alzheimer’s disease. Our idea is to determine whether there are safe gut metabolites that could be repurposed for Alzheimer’s treatment. Our lab has screened 400 commercially available gut metabolites and tested them using our stem cell-derived neuron model. We identified a few interesting ones, including a very common metabolite, vitamin K2, which significantly reduced tau hyperphosphorylation in the stem cell-derived neuron model. So, based on this preliminary data, we plan to expand our research to better understand the protective mechanisms of the microbiome-gut-brain axis in Alzheimer’s disease, which we think will produce new prevention and treatment strategies. We’re collaborating with Mark Brown, PhD, Director of Research at Cleveland Clinic’s Center for Microbiome and Human Health, who has gut microbiome expertise, and Jessica Caldwell, PhD, Director of the Women’s Alzheimer’s Movement Prevention Center at Cleveland Clinic.
If you’re successful in the research that these grants are funding, what impact do you think you’ll make on Alzheimer’s disease?
I cannot estimate what the outcome will be, but my goal or even my dream in the next few years is to test a drug we identify from our big data or multi-omics approach in a clinical trial. I really want to develop a new precision medicine treatment approach for this challenging disease.
Advertisement

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

Large NIH-funded investigation is exploring this understudied phenomenon

Observational evidence of neuroprotection with GLP-1 receptor agonists and SGLT-2 inhibitors

Genomic study lays groundwork for insights into potential biomarkers and therapeutic strategies

Proteins related to altered immune response are potential biomarkers of the rare AD variant

Alzheimer’s studies delve into sex-related variances in the expression of the disease

Validated scale provides a method for understanding how lifestyle may protect against Alzheimer's

Collaborative approach may reduce distress caused by neuropsychiatric symptoms