Could these be the culprits in the increasing incidence of kidney stones?

By Aaron Miller, PhD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The associations between urinary stone disease (USD), oxalate and the microbiome are well known. Microbial oxalate metabolism in the gastrointestinal tract involves a varied, functional microbial network that includes multiple species of oxalate-degrading bacteria, along with other functional groups of bacteria that include acetogenic bacteria, methanogenic bacteria, sulfate-reducing bacteria, and others.
Given that diet and antibiotic use strongly influence the microbiome, we sought to determine how antibiotics and diet influence microbial oxalate metabolism in the gut microbiota.
Both antibiotics and a high-fat, high-sugar (HFHS) diet significantly and persistently inhibited microbial oxalate metabolism by the gut microbiota in a mouse model that had received a fecal microbial transplant from the oxalate-consuming wild rodent, Neotoma albigula.
The gut microbiota from N. albigula exhibits robust oxalate-degrading capabilities resulting from a wide network of bacteria, which we have previously shown to be transferable to other rodent species.
To determine how antibiotics and a HFHS diet affect microbial oxalate metabolism, Swiss-Webster mice (SWM) implanted with the fecal microbial community from N. albigula, were subsequently given no additional treatment, three days of cefazolin antibiotics, a HFHS diet for 12 days, or both antibiotics and a HFHS diet. Urine and feces were collected every three days to track changes to urinary and fecal oxalate and the fecal microbiota.
Advertisement
To determine how the HFHS diet and antibiotics affected the functional microbial oxalate specifically, we first identified the group of bacteria that were significantly different between animals that received a fecal microbial transplant and no other treatment (NALB). We compared these with SWM that were implanted with their own fecal microbiota.
The difference in oxalate metabolism in these two groups is specifically the result of the differences in the gut microbiota. Following identification of the group of bacteria, we specifically tracked this network over a 12-day period by determining the co-occurrence network of these bacteria, an indication of a cohesive microbial network.
What we found was that the loss of oxalate metabolism resulting from antibiotics was specifically due to an acute loss of bacteria. However, while the gut microbiota returned to its post-transplant baseline, oxalate metabolism did not recover. In contrast, the loss of oxalate metabolism in the HFHS group may have been in part due to some loss of bacteria, but was attributed more to a shift in overall community composition and associated physiology.
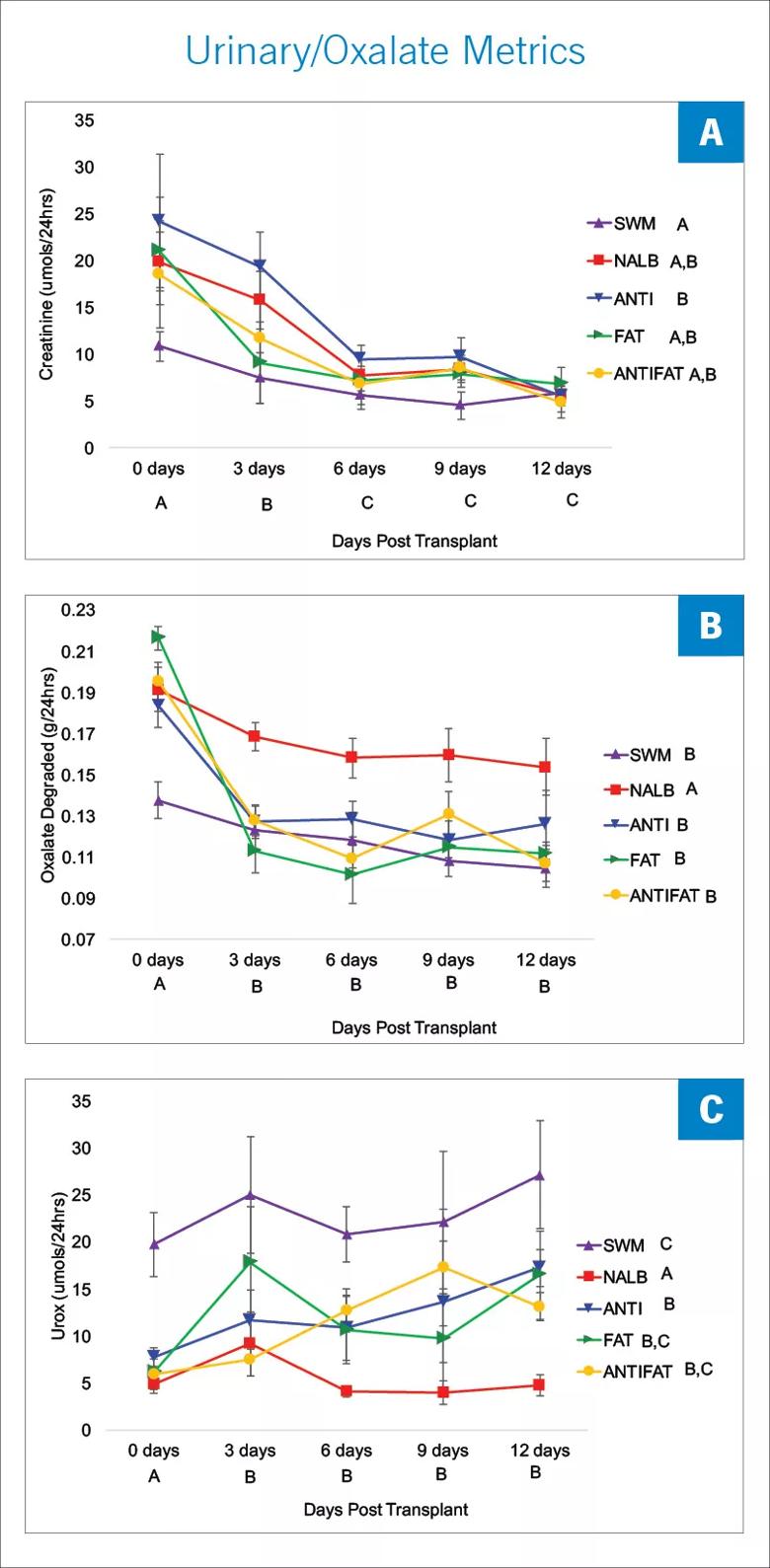
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a2148e7c-0d00-44c5-9ed6-83f55c9fd860/18-URL-824-QD-Miller-High-Fat_jpg)
Each time point represents the average daily value for the three-day interval. A) Urinary creatinine excretion; B) total microbial oxalate metabolism (oxalate consumed minus oxalate excreted); and C) urinary oxalate excretion (Urox). Letters indicate statistically significant differences either by treatment group (in legend) or by time point (X-axis) as determined by a repeated measures ANOVA and post-hoc Tukey’s Honestly Significant Difference analysis.
Advertisement
The results of our study show that both antibiotics and HFHS diet have a significant, negative impact on oxalate metabolism. Oxalate, present in 80 percent of kidney stones, is a significant contributor to the formation of stones and is often a target for therapy to prevent recurrent episodes of USD.
Our study suggests that one possible driver of the rapidly increasing incidence of USD is the shift in the form and function of the gut microbiota associated with antibiotic use and changes in dietary patterns by the American population.
Future work will focus on the restoration of the functional microbial networks associated with oxalate metabolism, through dietary and lifestyle changes, along with the development of novel probiotics.
Dr. Miller is a project scientist with Lerner Research Institute and Glickman Urologic & Kidney Institute. His work is funded by the National Institutes of Health and by Cleveland Clinic’s research program committee and Lerner Research Institute.
Suggested reading
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity

Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time

Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology