Multicenter pivotal study may lead to first endovascular treatment for the ascending aorta
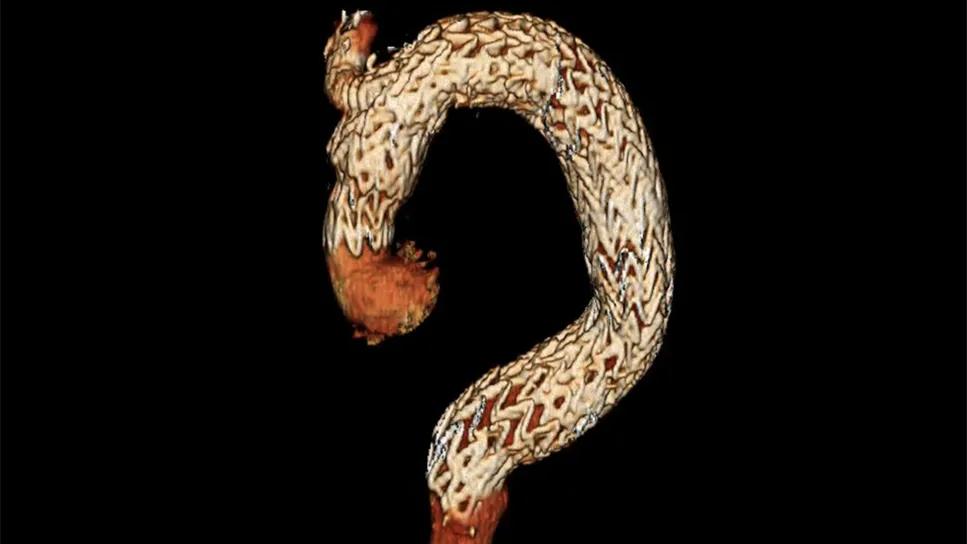
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ba982c00-9204-486a-9c50-04fbd3e96148/aorta-stent-graft)
stent graft in ascending aorta
A first-of-its-kind ascending aorta stent graft (ASG) is currently being evaluated in the pivotal ARISE II trial. The investigation comes on the heels of the completed early feasibility ARISE I study, in which the novel endovascular device was implanted in 19 patients with Stanford type A aortic dissection at nine U.S. sites.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Updates on the ARISE trials were presented by Cleveland Clinic staff at the 104th annual meeting of the American Association for Thoracic Surgery (AATS) earlier this year. “We’re in an exciting period of aortic management, with significant advances in monitoring and treatment on the horizon,” says cardiothoracic surgeon Patrick Vargo, MD, Cleveland Clinic’s site principal investigator in the ARISE trials.
The ascending aorta can be viewed as the final frontier of thoracic endovascular aortic repair (TEVAR), according to Eric Roselli, MD, national principal investigator of ARISE I and II and Chief of Adult Cardiac Surgery at Cleveland Clinic. Until recently, all endovascular stents for the aorta were designed for the descending or abdominal portions, which are straighter than the ascending aorta and have less dynamic pulsatility because of their greater distance from the heart.
“The ascending aorta has always posed special challenges to endovascular intervention,” says Dr. Roselli, who also serves as Cardiac Surgery Director of Cleveland Clinic’s Aorta Center. “We’re now making progress in minimally invasive alternatives to open surgery for our high-risk patients.”
The device studied in both ARISE trials is the GORE® ASG (Ascending Stent Graft). This investigational device is shorter than stents used in other parts of the aorta and is designed to articulate so it can foreshorten along the inner curve as it is deployed, allowing conformance to the shape of the vessel. “This purpose-driven design allows for incredibly precise deployment to avoid coronary obstruction and valve impingement, problems that plague other endovascular stents deployed off-label in the ascending aorta,” Dr. Roselli says.
Advertisement
The ARISE I single-arm study (J Endovasc Ther. 2023;30[4]:550-560) implanted the ASG in 10 patients with DeBakey type I disease (ascending aorta and aortic arch) and nine with type II disease (ascending aorta only). Patients were 58% female and had a mean age of 76 (range, 47-91). All were at high surgical risk, with 84% having acute dissection. Patients with severe aortic insufficiency or previous thoracic aortic surgery were excluded, as were those whose entry tear was less than 2 cm above the coronary ostia.
Thirty-day major adverse cardiovascular and cerebrovascular events occurred in five patients (26%), consisting of three deaths (16%), one disabling stroke (5%) and one myocardial infarction (5%).
Dr. Vargo notes that acute ascending aortic dissection is associated with a reported 58% in-hospital mortality rate if repair is not performed. “The takeaway from ARISE I is that ascending aortic dissection can be treated with endovascular stent placement with high technical success and acceptable safety in high-risk patients who are not surgical candidates,” he says.
ARISE II (NCT05800743) is recruiting 370 high-risk patients at 27 sites in the U.S. who have nonacute type A pathologies of the ascending aorta and arch (e.g., fusiform and saccular aneurysms, pseudoaneurysms, chronic type A dissections and other isolated lesions). Patients are undergoing TEVAR with ASG alone or ASG plus a thoracic branch endoprosthesis and will be compared with similar patients from a high-risk open surgical registry.
Advertisement
Primary outcome measures (successful implantation, graft patency, absence of reintervention, and absence of major adverse cardiovascular and cerebrovascular events) are being assessed at 30 days. Patients will be followed periodically through 60 months. The primary completion date is expected to be late 2029.
“Ascending stent grafting is an exciting development for the treatment of patients with type A aortic dissection and other ascending aortic pathologies who are at high surgical risk,” says Vidyasagar Kalahasti, MD, a staff cardiologist in Cleveland Clinic’s Aorta Center. “ARISE II is a very important and pivotal study to potentially expand its use in other ascending aortic pathologies after demonstration of technical feasibility in the ARISE I trial.”
Drs. Roselli and Vargo note that development of the ASG was aided by a collaborative research project that Dr. Roselli helped launch in 2016 known as MATADORS (Multidisciplinary Study of Ascending Tissue Characteristics and Hemodynamics for the Development of Novel Aortic Stentgrafts).
“MATADORS began in part to better understand the biomechanics and boundary conditions of the ascending aorta for devices like the one being used in the ARISE trials,” Dr. Roselli explains. “It also has led to important discoveries in the realm of basic and translational research around the mechanisms of disease involving the thoracic aorta.”
One such discovery was the focus of a presentation by Cleveland Clinic cardiac surgery research fellow Benjamin Kramer, DO, PhD, that won the 2024 AATS Nicholas T. Kouchoukos Award for Outstanding Aortic Symposium Manuscript. This prospective translational research led to the identification of aggrecan, a large proteoglycan, as a blood biomarker of diseased aortas.
Advertisement
In a healthy aortic wall, aggrecan is deposited along with collagen fibers and smooth muscle cells in an orderly array that contributes to the vessel’s integrity and elasticity. In prior studies, Cleveland Clinic investigators compared healthy to diseased ascending aorta samples and found that the latter had dramatically increased amounts of aggrecan and that the orderly structure was disrupted, likely contributing to the aortas’ predisposition to type A dissections.
The new research discovered that aggrecan can be detected in the blood, with patients about to undergo surgery for ascending aortic aneurysm or dissection having significantly higher levels than those undergoing coronary artery bypass grafting or healthy controls.
“The identification of this blood biomarker associated with aortic wall fragility has the potential to lead to better surveillance of patients with aortic aneurysm,” Dr. Vargo notes. “This could help us determine the appropriate time to intervene beyond aneurysm size.”
Advertisement
Advertisement
New Cleveland Clinic data challenge traditional size thresholds for surgical intervention
Study finds comparable midterm safety outcomes, suggesting anatomy and surgeon preference should drive choice
New review distills insights and best practices from a high-volume center
Blood test can identify patients who need more frequent monitoring or earlier surgery to prevent dissection or rupture
Recent Cleveland Clinic experience reveals hundreds of cases with anatomic constraints to FEVAR
Residual AR related to severe preoperative AR increases risk of progression, need for reoperation
Large cohort study finds significant effect in men and nonsmokers
Impacts include major emphases on multidisciplinary teams, shared decision-making