Phase 1/2 clinical trial builds on encouraging pilot study
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ab953d95-b14a-4522-a875-7611d9cfc77b/18-NEU-987-Transcranial-Stim-Grant-650x450_jpg)
18-NEU-987-Transcranial-Stim-Grant-650×450
Cleveland Clinic’s Lerner Research Institute has been awarded $2.5 million by the U.S. Department of Defense (DoD) to study a promising new rehabilitation therapy for patients with long-standing incomplete cervical spinal cord injury.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The research, led by Ela Plow, PhD, PT, an investigator with Cleveland Clinic’s Department of Biomedical Engineering and Department of Physical Medicine and Rehabilitation, involves transcranial magnetic and direct current stimulation (tDCS) applied to the scalp of patients, targeting areas in the brain that control muscles of the upper extremities weakened by spinal cord injury.
The new study builds on an earlier pilot study, also directed by Dr. Plow, that found promising results from using the noninvasive technique over 10 sessions in patients with chronic incomplete tetraplegia, allowing many to regain function.
“We are delighted to have the opportunity to validate and extend our previous findings in a large clinical trial,” says Kelsey Potter-Baker, PhD, lead author of the pilot investigation and a colleague of Dr. Plow’s in Cleveland Clinic’s Department of Biomedical Engineering. “We look forward to offering this promising noninvasive method to more people and ultimately see it become part of standard rehabilitation therapy.”
Dr. Potter-Baker will be reporting the final results of the pilot study in a platform presentation at the Academy of Spinal Cord Injury Professionals (ASCIP) conference in September 2018 (preliminary results of the study were presented at last year’s ASCIP conference — see this earlier Consult QD post for details).
The pilot study involved 18 patients randomly assigned to either tDCS or sham stimulation, in addition to massed practice training in both groups. Three months after the patients completed 10 sessions, the following outcomes were found in tDCS-treated subjects relative to controls:
Advertisement
This degree of improvement in patients more than one year after injury is highly significant, according to Dr. Potter-Baker, who adds that some patients had life-changing functional gains. She describes one woman whose goal was being able to dress herself with a “hoodie” sweatshirt; by the end of treatment, she could lift her arms over her head to do so.
“Some patients who benefited from tDCS were 15 years post-injury,” she says. “It’s amazing to see gains in patients who had been stable for so many years or even regressing.”
The complete results of the pilot study are expected to be published in early 2019.
The new DoD-funded investigation is a phase 1/2 randomized, controlled, double-blind trial to be conducted at multiple sites, including Cleveland Clinic, the Louis Stokes Cleveland VA Medical Center, MetroHealth Medical Center in Cleveland and the Kessler Institute for Rehabilitation in New Jersey. Forty-four enrollees are expected over four years.
The new trial will be similar in design to the pilot study, except that therapy will be extended to 15 sessions (generally conducted about three times a week). Follow-up assessment will again be done three months after the last session.
As part of the phase 1/2 study design, safety of the therapy will also be evaluated. Dr. Potter-Baker says no safety issues were identified in the pilot study.
Dr. Potter-Baker describes two important new technological developments that are accelerating research in tDCS therapy:
Better-targeted tDCS delivery system. The conventional system involves delivering current via kitchen sponge-sized devices strapped to the head. Dr. Plow’s group is now experimenting with a transcranial electrical stimulation device with button-sized anodes that better focalize current, allowing more targeted delivery to desired areas (see Figure below).
Advertisement
Improved imaging with HD-Explore. The new study will be using more-advanced imaging software called HD-ExploreTM (Soterix Medical), which provides better modeling of brain current flow during tDCS. This will enable investigators to better understand how the flow current is targeting the cortical pathways dedicated to patients’ weak muscles.
Dr. Potter-Baker explains that these new developments are especially important because her group’s pilot research found that improvements in upper limb mobility were associated with the amount of current targeting motor cortical areas dedicated to the weak muscles. She anticipates that the new technology will lead to better targeting capability, which will help optimize outcomes.
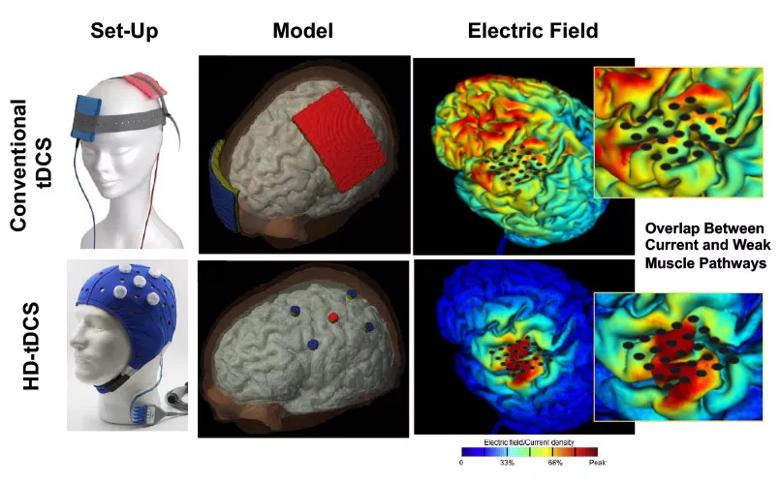
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/62d9970e-1a6c-4428-bdf2-9ca94b481ff9/18-NEU-987-Transcranial-Stim-Grant-inset_jpg)
Figure. Comparison of conventional tDCS (top row) with tDCS using HD-Explore software and a new delivery system using button-sized anodes. The conventional method results in widespread current distribution that doesn’t optimally target weak muscle pathways, whereas HD-tDCS focalizes current under the anode and the targeted pathways. Top left image courtesy of neuroCare Group. Bottom left image courtesy of Soterix Medical.
Advertisement
Advertisement
First full characterization of kidney microbiome unlocks potential to prevent kidney stones
Researchers identify potential path to retaining chemo sensitivity
Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time
Investigators are developing a deep learning model to predict health outcomes in ICUs.
Preclinical work promises large-scale data with minimal bias to inform development of clinical tests
Cleveland Clinic researchers pursue answers on basic science and clinical fronts
Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches
Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology