Despite its longevity, this foundational operation keeps getting refined
By Laurice Bakaeen, PharmD; Jaikirshan Khatri, MD; and Faisal G. Bakaeen, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
This article is an abridged version of a review we recently published in Cleveland Clinic Journal of Medicine (CCJM) (2021;88:295-303). Most of the original review is retained, just with a bit less detail on some finer points to make for a briefer, more practical read for this Consult QD format. As a result of this abridgement, the citation of references is not comprehensive and not always sequential, and the references list is not included here. For the full-length, fully referenced version of this review, see the open-access original version on the CCJM website.
Coronary artery bypass grafting (CABG) has been performed for more than 50 years. Although the operation is increasingly being used for older and higher-risk patients, outcomes have improved substantially over time. The surgery has developed beyond a “cookie-cutter” generic cardiac operation, and the use of a multidisciplinary, experienced heart team approach has become important.
In 1968, Cleveland Clinic established CABG as the standard of care for obstructive coronary artery disease (CAD).3 Two years later, a Cleveland Clinic team led by René Favaloro reported on the workup and favorable outcomes of more than 300 patients who underwent “venous autograft reconstruction” with appropriate follow-up.4
All-venous-conduit CABG reigned from 1968 until January 1986, when Loop et al5 demonstrated improved graft patency and a 10-year actuarial survival with internal thoracic artery (ITA) grafts compared with saphenous venous grafts anastomosed to the left anterior descending (LAD) coronary artery.
Advertisement
Pursuit of improved outcomes has intensified in the current era of public reporting. Perioperative mortality rates have been reported nationally at 2% (and at < 1% at some centers of excellence).3 But beyond perioperative mortality and morbidity, interest in improving long-term outcomes has grown. Debate continues about the use of bilateral ITA grafting and other multiarterial grafting strategies. Minimally invasive options and robotic assistance are also evolving.6
Coronary angiography remains the gold standard for diagnosing CAD.9 Optical coherence tomography, intravascular ultrasonography, fractional flow reserve, cardiac CT angiography and cardiac MRI are newer diagnostic methods that provide more than a simple subjective visual estimation of coronary narrowing; they provide information on granular anatomic and physiologic features of coronary lesions and the downstream effect on the myocardium.
Three main factors should be considered when deciding on an intervention strategy for CAD.
1) Disease stability. CAD stability and presentation — i.e., ST-elevation myocardial infarction (STEMI), non-STEMI or stable angina — are factored into the management algorithm. Percutaneous coronary intervention (PCI) is the treatment of choice for STEMI; for non-STEMI and stable angina, recommendations are more nuanced. In patients with stable CAD and low-risk anatomic features, PCI has failed to show convincing evidence of benefit beyond a modest reduction in angina.14,15 Comparisons of CABG and medical therapy are dated, and emphasis now is on complementary rather than competing therapies.16,17 Medical treatments (e.g., high-intensity statins, dual antiplatelet therapy, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and novel glucose-lowering agents) are transforming primary and secondary cardiovascular prevention in patients with stable angina, resulting in reduced event rates in recent years.18 Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for patients with type 2 diabetes mellitus and renal impairment are associated with reduced disease progression and recurrent ischemic events.19
Advertisement
2) Procedural risk and patient comorbidities. CABG risk is most commonly and reliably estimated by the Society of Thoracic Surgeons risk calculator, which estimates the risk of perioperative mortality and major morbidity.20 The latter includes stroke, with about a 1% perioperative rate, which is slightly higher than the risk associated with PCI.21 Advanced age is an important risk factor for stroke and periprocedural mortality, but it should be considered in the context of other risk factors when choosing between therapies. Risk models perform well at a population level but are limited for estimating risk for individuals, particularly for those with rare comorbidities or unique risk profiles. Patients with significant baseline comorbidities, frailty (not captured by the Society of Thoracic Surgeons calculator) and reduced life expectancy are best suited for PCI.
3) Atherosclerotic burden and disease complexity. CAD complexity is often assessed using the Synergy Between PCI With TAXUS and Cardiac Surgery (SYNTAX) trial score,22 which is incorporated in the American College of Cardiology/American Heart Association criteria for treatment selection. A heavy atherosclerotic burden favors CABG over PCI.23
Historically, the mortality rate in untreated left main CAD is about 50% at three years.24 It is a heterogeneous condition that may involve the ostia, midshaft, bifurcation or trifurcation. The specific areas involved affect the feasibility and success of PCI but have no bearing on CABG success or durability.
Advertisement
The role of PCI versus CABG in left main disease is controversial, with two recent trials showing seemingly different findings. However, neither favored PCI over CABG.16
About 10% of STEMIs involve the left main coronary artery. In STEMI or hemodynamic instability, PCI is the treatment of choice. In non-STEMI and stable ischemia, the American College of Cardiology/American Heart Association guidelines give the highest recommendation for CABG for all SYNTAX levels27; PCI is recommended at this level only for low-risk SYNTAX scores.
Left main and multivessel CAD are treated as different entities in the literature, even though less than 15% of lesions are isolated left main disease. SYNTAX 10-year data show an all-cause mortality benefit for CABG over PCI in patients with three-vessel disease (21% vs. 28%).28
Current guidelines recommend CABG over PCI for multivessel CAD in patients with diabetes and for those with left ventricular dysfunction.27 Even for severe left ventricular dysfunction, CABG is associated with improved long-term outcomes, including survival, compared with PCI for patients with indications for CABG and who can tolerate the stress of surgery.29
Conduit selection is a current topic of debate.
Saphenous vein. Attrition of the saphenous vein graft, the Achilles’ heel of CABG, occurs in phases. The first phase is nearly immediate and likely related to a technical factor. This can be avoided with intraoperative evaluation of the bypass graft. Transit-time flow meters can identify low graft flows due to thrombosis, kinking, conduit dissection, coronary dissection or anastomosis stenosis, all of which are potentially correctable.31 Subsequent phases of vein graft failure include intimal hyperplasia and atherosclerosis. Saphenous vein graft attrition rates of 1% to 2% per year for the first six years and 4% per year for the next decade have been reported.32
Advertisement
Arteries versus veins. Angiography data reported over a 15-year period revealed that coronary territories bypassed with arteries had less disease progression compared with territories bypassed with veins.33 The internal elastic lamina of arterial grafts protects them from disease progression. Native coronary disease is also protected by arterial grafts for unclear reasons, but possibly due to the downstream effect of vasoactive signals.34
ITA and radial artery grafts. At 15 years, right ITA graft patency is reported to be more than 90% and left ITA graft patency more than 95%.35 The Society of Thoracic Surgeons guidelines13 recommend the following:
In 2019, five-year data from the RADIAL study showed a benefit for using the radial artery rather than the saphenous vein for graft occlusion and target revascularization.36 Rates of myocardial infarction and repeat revascularization were also superior for radial arteries, and a mortality benefit was reported in a follow-up study.37
Evidence favors multiarterial options. In 2019, the Arterial Revascularization Trial 10-year intention-to-treat data showed no difference in survival or event-free survival for bilateral versus left ITA. However, a 14% crossover rate, excellent medical compliance and a radial artery conduit in more than 20% of patients possibly clouded the results.38 A post hoc as-treated analysis showed improved mortality and major adverse cardiac and cerebrovascular events with multiple arterial grafting. Additionally, a five-year post hoc analysis found that radial artery grafting improved outcomes in both groups.39
Since 2001, five major systematic reviews and one meta-analysis found that bilateral ITA grafting offered a survival advantage over left ITA grafting, including long-term survival, reduced hospital mortality, reduced cerebrovascular accidents and reduced revascularization.38
Despite evidence of the benefits of multiple arterial grafting and the professional association recommendations to encourage its use, only a small percentage of patients undergoing CABG in the U.S. receive multiarterial grafts. Reasons for this include additional technical complexity, prolonged operative times and potential for complications.40
Optimizing success of multiarterial grafts. Multiple arterial grafting (Figure 1) is not without its nuances, including conduit choice and intended target coronary vessel. For example, radial artery grafts are best used to bypass severely diseased target vessels to minimize competitive flow and optimize graft patency.13 The myocardial mass supplied by a diseased vessel is also critically important. Important target vessels extend more than 75% of the way to the apex of the heart, and matching these important vessels with the second arterial graft has a long-term mortality benefit.44
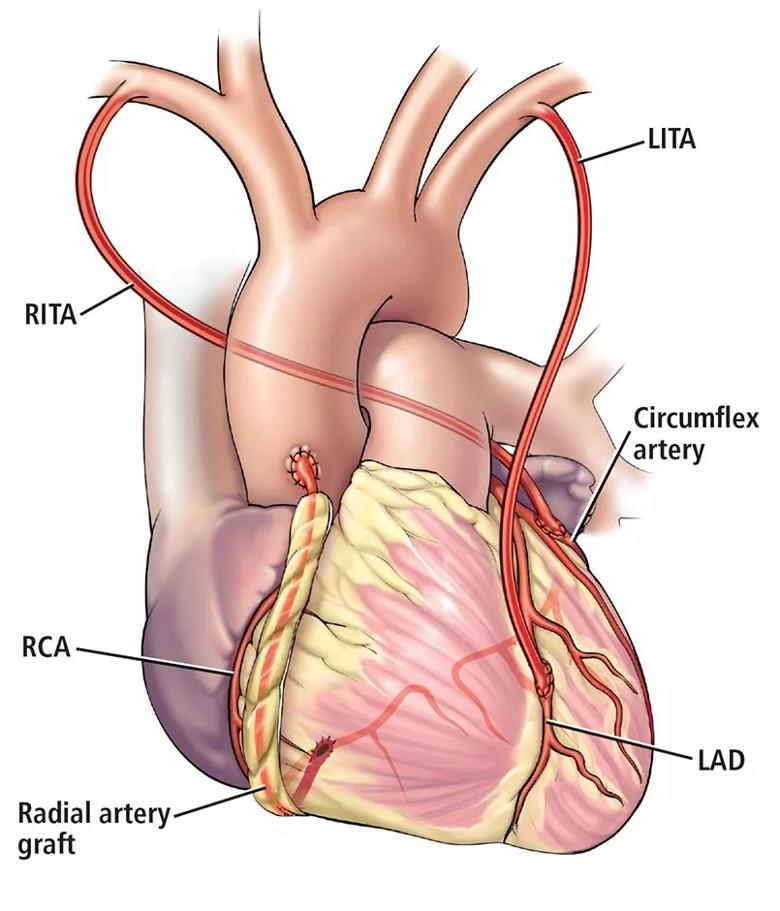
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e17e9102-395b-43ff-9bed-a3f94808375c/21-HVI-2287616-Inset1-805x940-1_jpg)
Figure 1. An example of multiarterial CABG. The left internal thoracic artery (LITA) is used to bypass the left anterior descending artery (LAD), the right internal thoracic artery (RITA) to bypass the circumflex artery, and the radial artery to bypass the right coronary artery (RCA).
The importance of an experienced coronary surgeon in decision-making and the performance of CABG cannot be overstated.7,8 A specific volume-outcome relationship has been described for bilateral ITA grafting.46 The increased risk associated with surgery for complex revascularization procedures such as redo CABG is well documented47 but is mitigated by surgical expertise.48 In addition, a focused interest in CABG facilitates innovation and the development of less invasive approaches.
Off-pump CABG avoids use of cardiopulmonary bypass and is physiologically less invasive than traditional on-pump CABG. Off-pump CABG can benefit select high-risk patients not typically enrolled in trials. Surgical experience is critical in mitigating reduced graft patency and incomplete revascularization associated with off-pump CABG.49 Widespread adoption is ill-advised, and use of off-pump CABG has in fact declined.
Robotic CABG accounts for less than 1% of CABG operations in the U.S.6 Data supporting use of these procedures outside of select specialized centers are currently limited. Technology is lagging, and it is difficult to teach robotic multiarterial CABG and reliably achieve complete revascularization.
Hybrid CABG uses robotic or minimally invasive left ITA harvest with a direct hand-sewn left ITA-to-LAD artery anastomosis through a minithoracotomy (Figure 2). Non-LAD artery stenosis is then addressed with drug-eluting stents. Theoretical benefits are lower occurrence of stroke, decreased infection, sternal sparing, fewer transfusions and faster recovery.
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5ea1a29f-54f6-442f-ad97-7c05f33be462/21-HVI-2287616-Inset2-805x652-1_jpg)
Figure 2. Example of minimally invasive CABG, performed through a small left thoracotomy incision, in which the left internal thoracic artery is bypassed to the left anterior descending artery without use of a heart-lung machine.
Enhanced recovery after surgery relies on evidence-based protocols designed to improve outcomes and cost savings based on rigorous data review and protocol development.50 Postoperative goal-directed hemodynamic resuscitation algorithms reduce 30-day major adverse cardiovascular events in high-risk patients.51 Similarly, fast-track early extubation protocols decrease time on a ventilator. Shorter extubation times are associated with decreased length of stay and hospital cost.52
Optimal medical management for secondary prevention and improved long-term outcomes after CABG has been increasingly recognized.54 Discharge prescriptions for beta blockers and statins are process measures tracked by the Society of Thoracic Surgeons as part of its program quality ratings. The benefits of beta blockers include a potential decrease in long-term mortality after CABG.55 In patients receiving radial artery grafting, use of antispasmodic medications, including calcium channel blockers, is associated with improved outcomes.56 Statin use after surgery is associated with decreased readmissions and late death from myocardial infarction or stroke.57
Dual antiplatelet therapy is now recommended for six months in patients with acute coronary syndrome undergoing CABG. Additionally, in patients who had coronary stenting prior to CABG, dual antiplatelet therapy may prolong stent patency and prevent thrombus development and propagation.58
Comprehensive rehabilitation programs have been developed to prevent readmissions and improve treatment compliance and quality of life after discharge. Medication adherence dramatically improves outcomes regardless of coronary revascularization strategy.59
References are available in the full-length original version of this article in Cleveland Clinic Journal of Medicine (2021;88:295-303).
Advertisement
Modified-Bentall single-patch Konno enlargement (BeSPoKE) optimizes hemodynamics, facilitates future TAVR
Cleveland Clinic’s new dedicated program offers nuanced care for a newly recognized cardiovascular risk factor
Scenarios where experience-based management nuance can matter most
Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics
How Cleveland Clinic is using and testing TMVR systems and approaches
NIH-funded comparative trial will complete enrollment soon
How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR
Optimal management requires an experienced center