Treatment based on next generation sequencing shows promise
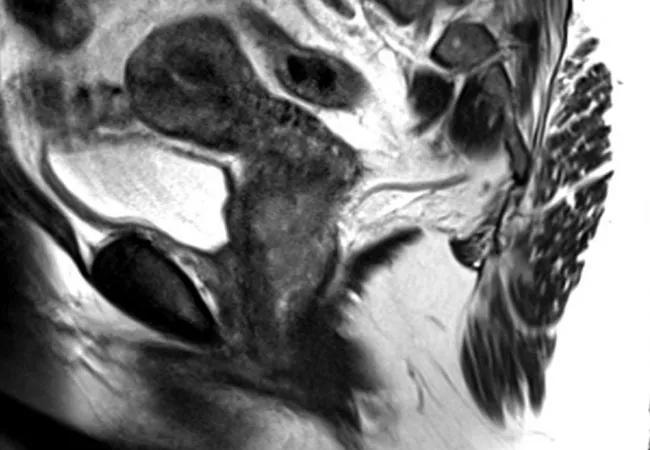
A 58-year-old woman presented to Cleveland Clinic with post-menopausal bleeding. Gynecologic oncologist Peter Rose, MD, ordered an MRI which showed an enhanced cervical mass. Further testing uncovered small bilateral cervical lymph nodes with mild FDG uptake SUV of 2.7, concerning for metastases.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Because of the rarity of neuroendocrine carcinomas of the cervix, occurring in 0.9% of cervical cancers, treatment is often modeled after the more common neuroendocrine small cell carcinoma of the lung.
“We looked at a study from 2014 that reported on 280 neuroendocrine carcinomas of the cervix. These patients presented with advanced stage, large tumors,” says Dr. Rose. “Not surprisingly, patients experienced a poor survival rate. Researchers from that study reported a three-year survival rate of 9.4%.”
Because of the rarity of these cases, there are no prospective trials addressing clinical management. Patients are usually treated with carboplatin and etoposide. When this is ineffective, oral topotecan is used as a second-line therapy.
In this case, the patient was initiated on chemotherapy with carboplatin at an AUC of 5 Day 1 and etoposide 100 mg/m squared days 1 through 3 every 21 days for 11 cycles.
“Another CT scan after cycle 3 showed that her disease was overall stable and even decreased in size,” says Dr. Rose. “After nine cycles of therapy, her disease remained stable and she agreed to undergo Foundation One testing to look for potential therapeutic targets.”
Foundation One testing, which provides genomic insights to providers, showed the following genomic alterations: BRCA2 loss exons 17-27, KMT2C (MLL3) splice site 1012 + 1G > A – subclonal, NOTCH2 Q1634 and PARK2 loss exons 1-10. Potential therapies included the PARP inhibitors niraparib, rucaparib and olaparib. No clinical trials or approved therapies were available for the other mutations. Because the patient had a somatic mutation, and only one of the three FDA-approved PARP inhibitors had an indication for somatic mutation, therapy with rucaparib 600 mg orally BID was prescribed.
“Our team undertook next generation sequencing of this patient’s tumor with a goal of finding an actionable mutation,” says Dr. Rose. “Previous studies utilizing target-directed therapy against the BRCA mutation have not been reported in small cell carcinoma of the cervix.”
In a 2014 study published in Gynecologic Oncology, researchers found that small cell carcinoma from different organs within the female genital tract share the same histopathologic characteristics that resemble those of small cell lung carcinoma. The expression of at least one immunohistochemical neuroendocrine marker was a common finding.
Overall, studies evaluating the benefit of genomic profiling are lacking.
Advertisement
However, in 2015, a team of researchers at Cleveland Clinic conducted a prospective study in 250 patients with non-gynecologic solid tumors. Of the 223 evaluable samples, 49% of patients were recommended a specific therapy. Due to lack of clinical trial access or insurance coverage, only 11% received therapy.
One clinical trial is trying to change that. NCI-MATCH, also known as MATCH, is for adults whose tumors have progressed on standard treatment or rare cancers for which there is no standard treatment. The trial evaluates molecular profiling and then provides therapy at no cost to the patient. Results are slowly being reported.
Arm I studied the drug taselisib in 65 patients with mutations in the PIK3CA gene. There were no objective responses to the drug; however, 24% of the patients had progression-free survival of greater than six months.
Dr. Rose and colleagues published this case study in Gynecologic Oncology Reportsand hope this patient’s recent treatment plan using Foundation One testing results in more research, clinical trials and success stories. He notes, “The fact that our patient has received 30 months of therapy and imaging every three months and the disease remains stable is quite remarkable.”
Feature image: MRI of the pelvis, sagittal section of the uterus and cervix demonstrating cervical tumor. Republished with permission from Elsevier.
Advertisement
Advertisement

Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors