Initial pilot study findings suggest a potential reduced need for cerebrospinal fluid testing
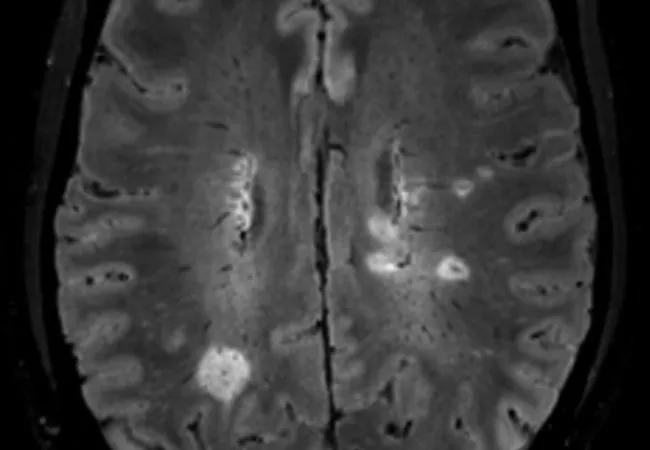
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/bc37e7c3-8ca5-4618-af53-3c3f0bfa1695/22-NEU-3409067_central-vein-sign_650x450_jpg)
22-NEU-3409067_central-vein-sign_650x450
Detection of the central vein sign on brain imaging showed similar accuracy as oligoclonal bands in cerebrospinal fluid for the diagnosis of multiple sclerosis (MS) in a retrospective analysis of the Central Vein Sign in the Early Diagnosis of MS (CAVS-MS) pilot study.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The finding, reported in a poster presentation at the 38th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS 2022), is a notable boost for the potential utility of the central vein sign, a blood vessel seen within lesions in white matter on fluid-attenuated inversion recovery (FLAIR) MRI sequences.
“The diagnostic performance of the central vein sign detected by FLAIR* was similar to that of OCB in CSF,” says the study’s first author, Karlo Toljan, MD, a neurology resident at Cleveland Clinic. “Using the central vein sign as a diagnostic criterion may one day decrease the need for invasive CSF investigations and enable earlier and more accurate MS diagnosis.”
The presence of OCB in CSF can serve to meet the requirement for dissemination in time in the 2017 McDonald criteria for diagnosing MS. However, the criteria were developed in patients with a typical presentation, and it is estimated that 20% of patients diagnosed with MS may have been incorrectly diagnosed, many times leading to unnecessary use of expensive and sometimes risky medications.
The central vein sign — best detected using FLAIR* (i.e., combining FLAIR with high-resolution contrast-enhanced T2*-weighted imaging for detection of central veins) — is increasingly recognized as a new biomarker that might improve diagnostic accuracy and may reduce the need for obtaining a CSF sample.
The large, prospective multicenter CAVS-MS study (NCT04495556), for which Cleveland Clinic is the coordinating center, is currently underway to evaluate the central vein sign’s potential utility for diagnosing MS (as summarized in a previous Consult QD post). The study is designed to include 400 patients with typical clinical presentations and atypical presentations. Patients will be followed clinically and undergo FLAIR* imaging and other diagnostic testing at baseline and at 24 months.
Advertisement
The pilot study for CAVS-MS was primarily designed to optimize and harmonize FLAIR* MRI — a technique that has been in development for several years — at 10 sites in North America. This subanalysis compared the sensitivity, specificity and positive predictive value of central vein sign quantification to OCB in CSF for diagnosing MS at baseline and 12 months. Post-gadolinium FLAIR* images were rated as either Select-3* or Select-6* — i.e., containing at least three or at least six central vein sign lesions per scan.
The cohort consisted of approximately 100 participants, of whom 53 had undergone CSF testing for clinical purposes in addition to MRI ratings for central vein sign; of this latter group, 24 patients (45%) met 2017 McDonald criteria for MS at initial presentation, and an additional three patients met the diagnostic criteria at 12-month follow-up.
Key results were as follows:
“In most patients, the central vein sign was concordant with OCB determination for dissemination in time,” says Daniel Ontaneda, MD, PhD, the principal investigator of the Cleveland Clinic site for the CAVS-MS trial and a neurologist with Cleveland Clinic’s Mellen Center for Multiple Sclerosis Research and Treatment.
Advertisement
Dr. Ontaneda emphasizes that the complete CAVS-MS trial will provide more definitive results, with a larger study population and longer follow-up period. He expects preliminary results to be available mid-2023 and final results in 2026.
“Will the central vein sign turn out to be a sufficiently specific biomarker that we can forego additional tests, including a spinal tap, in most patients?” asks Dr. Ontaneda. “This is the pressing question that we need to answer and is especially important for those with atypical presentations.”
He adds that he would like for neurologists to one day be able to diagnose MS easily and accurately based just on MRI results, similar to how cardiologists now diagnose heart disease using echocardiography rather than their past reliance on symptoms or exam findings, for example.
“The old way of diagnosing heart disease was analogous to how we currently use the McDonald criteria to diagnose MS,” he explains. “It involves a complicated set of rules and is not completely accurate if not applied correctly. I would ultimately like to have an imaging test with high sensitivity and specificity for MS, simplifying the process and ensuring a correct diagnosis.”
Image at top: Representative brain MRI with lesions showing the central vein sign.
Advertisement
Advertisement
Guidance from the largest cohort of SEEG-confirmed insular epilepsy patients reported to date
Ethical guidance provides guardrails so medical advances benefit patients
OCEANIC-STROKE results represent long-sought advance in secondary stroke prevention
Two studies from Cleveland Clinic may help advance the technology toward broader clinical use
Distinct MRI signature includes lesions beyond the corpus callosum, features predictive of vision and hearing loss
An argument for clarifying the nomenclature
An expert talks through the benefits, limits and unresolved questions of an evolving technology
Recommendations on identifying and managing neurodevelopmental and related challenges