
Expert advocates for a stepladder approach
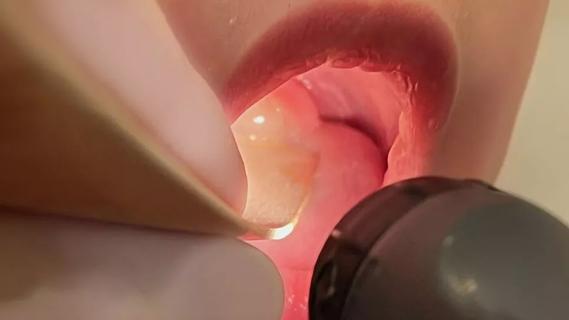
Prompt surgery was necessary when symptoms drastically increased
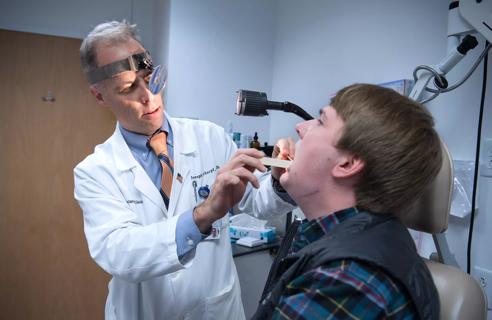
Strong communication with the patient and a thorough approach are essential
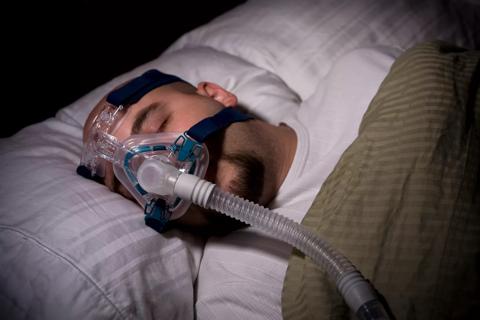
A review of conservative, pressure-based and surgical treatments for OSA
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy

Research on children with UHL explores the quality-of-life benefits and outcomes of cochlear implants

A look at how custom-fitted oral appliances work and when they’re a good fit for patients

With a wide scope of skills, comprehensive otolaryngologists care for patients of all ages in the community
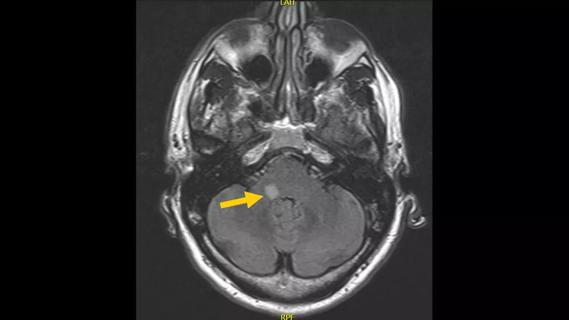
Subtle information gleaned from clinical examinations prompted concern
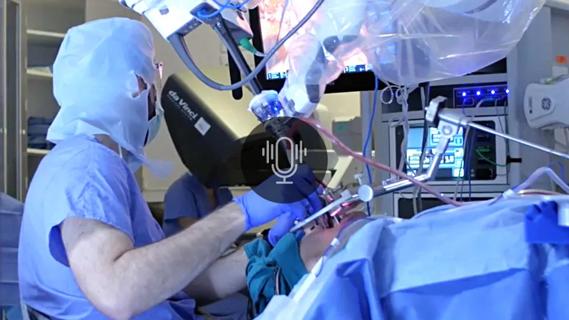
A new single-port system well-suited for oropharyngeal cancer treatment

Challenging case requires outside-the-box approach