Rare genetic disorder prevents bone mineralization
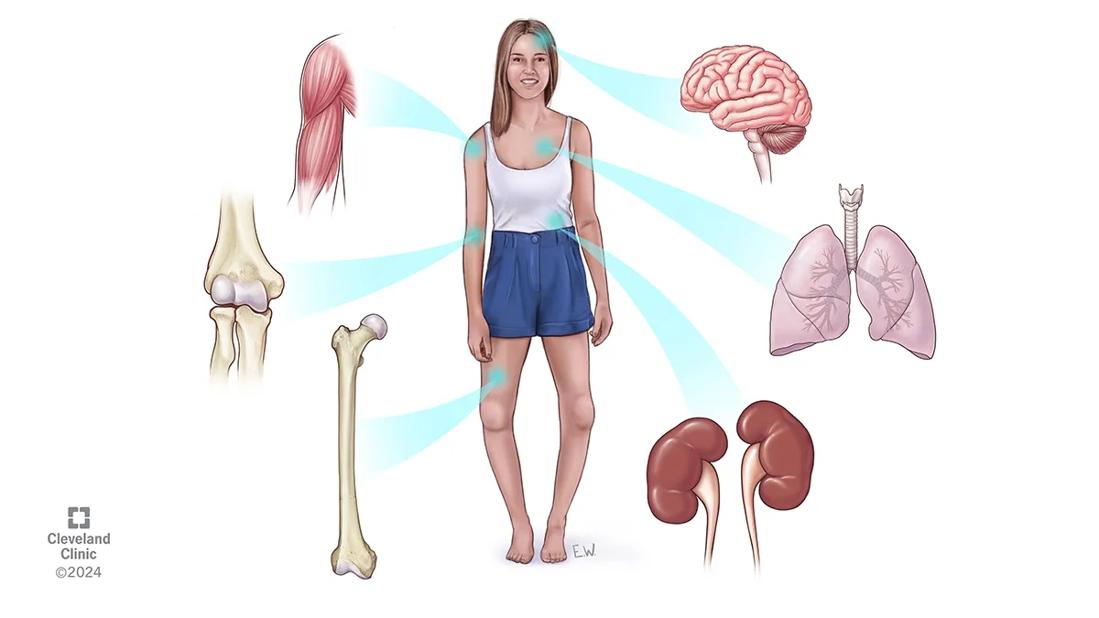
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2381bc59-be7e-4dcc-af3d-4ea8ec68751d/CQD_Hypophosphatasia-Clinic_24-EMI-5369634)
Illustration of hypophosphototasia symptoms
Hypophosphatasia (HPP) is a rare, sometimes debilitating genetic bone disorder caused by loss of tissue-nonspecific alkaline phosphatase (TNALP) activity. It often goes undiagnosed for years, and even after diagnosis, patients sometimes are advised against starting treatment.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
At Cleveland Clinic, Leila Khan, MD, Co-Director of Cleveland Clinic’s Calcium/Parathyroid Center, works with colleagues in genetics and endocrinology to assess and treat individuals with HPP — some of whom have been frustrated for years looking for the underlying cause of their symptoms.
HPP can cause a wide spectrum of musculoskeletal, renal, dental and neurological symptoms. Hallmarks include loss of teeth as well as non-traumatic bone fractures and skeletal malformations. While severe HPP is often diagnosed prenatally or in childhood, milder forms sometimes go undiagnosed because of vague symptoms and a generally low level knowledge of the condition. Among primary care physicians, there is little awareness of HPP, says Dr. Khan, and even within general endocrinology the disorder often goes unrecognized for years.
In 2015, the U.S. Food and Drug Administration granted breakthrough therapy designation for asfotase alfa, the first —and to date only — treatment for individuals with pediatric-onset HPP. That year, Dr. Khan treated a patient who was referred by her endocrinology colleagues and had been suffering from repeated bone fractures and low bone density. Asfotase alfa returned normal function.
“That person was finally able to put on her makeup, cut her steak, walk — do things that most of us take for granted,” Dr. Khan says. “Because of that case, we started writing up posters and presenting at national meetings.”
Since 2015, Dr. Khan’s team has treated about 20 people with the disorder, all of whom have experienced functional improvement after starting a regimen of asfotase alfa.
Advertisement

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/08c3b76a-87d5-4e3c-b276-1d762a99161e/CQD_Hypophosphatasia-Clinic-labeled_24-EMI-5369634)
The ALPL gene produces TNALP, which normally breaks down inorganic pyrophosphate (PPi). Loss-of-function mutations of ALPL cause accumulation of PPi, which impairs calcium-phosphate hemostasis and can cause damage to multiple organs. TNALP also facilitates vitamin B6 in crossing cell membranes. Extracellular accumulation of active B6 can result in neurological damage.
“TNALP deficiency causes a spectrum of clinical scenarios,” says Dr. Khan. “It causes some people to have short stature, muscle fatigue and fractures. Other people actually look and feel fine but have significant fatigue.”
At a conference, Dr. Khan experienced a simulation that allowed clinicians to feel the level of fatigue that afflicts some people with HPP. “You wear 40 pounds of equipment, and that’s how they actually feel,” she says.
Ideally, HPP is diagnosed in childhood — sometimes by a dentist who notices that a child has lost their baby teeth earlier than normal. For those who reach adulthood without a diagnosis, however, finding the reason for their symptoms can be difficult.
“These patients oftentimes will have been seen for fibromyalgia-like symptoms, and they've not really been noted to have low alkaline phosphatase,” says Dr. Khan. “They've been sent on their way, and it’s only when someone suspects HPP that they can look back at their medical history and see the symptoms that had pointed toward that.”
When HPP is suspected, Dr. Khan performs screenings that include review of:
Advertisement
Dr. Kahn also evaluates patients’ muscle strength.
For cases that are not clear cut, or if patients don't have an overwhelming number of symptoms, a genetic assessment may be done. But no finding of ALPL mutations doesn’t rule out HPP, says Dr. Khan. “A lot of the gene mutations are yet to be discovered.”
The enzyme replacement treatment asfotase alfa can be prescribed only for those with juvenile-onset HPP. The treatment is delivered by subcutaneous injection in weight-based dosage three times a week. Anecdotally, Dr. Khan has noted that people feel better after one to two months.
Patient follow-up generally takes place soon after treatment begins and again at six months and a year.
So far, she says, almost everyone she has started on the medication has continued it, but she adds “there's no hard and fast rule that it has to be continued, although it does make physiologically sense to be on enzyme replacement.”
In general, one of the main barriers to treatment has been perceptions about cost. In 2019, a New York Times report about the price of drugs for rare diseases featured a family with three members who had HPP and the union healthcare plan struggling to pay for their treatment. Some clinicians have dissuaded patients from seeking the drug because they believed the cost would have to be borne out of pocket. So far, however, Dr. Khan’s patients all have received insurance coverage.
The takeaway for physicians with patients showing symptoms consistent with HPP is to pursue diagnosis in collaboration with experienced peers.
Advertisement
“Patients with fibromyalgia-like symptoms and low alkaline phosphatase levels in their charts really should be screened for this,” says Dr. Khan.
It’s also important to collaborate with labs that understand the nuances of alkaline phosphatase levels, she says. “There are periods in people's lives when they have high alkaline phosphatase levels, such as in childhood or in a setting of a bone fracture or pregnancy. The reference ranges are different. So even if their alkaline phosphatase on some occasions has looked normal, one has to not automatically dismiss these cases.”
Advertisement
Advertisement
Recommendations on identifying and managing neurodevelopmental and related challenges
Phenotypic clustering study reveals four distinct disease trajectories
Oral medication reduces epistaxis and improves quality of life for patients with rare vascular disorder
A recently published case series highlights the broad range of laryngeal findings that can present among individuals with EDS
The disorder can greatly impact patients’ quality of life, but sclerotherapy and a multidisciplinary approach to care can be life-changing
Case study of radial-to-axillary nerve transfer for tumor-related deltoid nerve injury
Study also finds that 26% of children with cancer have mutations in DNA repair genes
National database study reveals insights into survival outcomes