Pivotal COSIRA-II multicenter U.S. clinical trial is underway
Patients with severe refractory angina who are ineligible for revascularization recently gained expanded access to a new therapy option: implantation with a coronary sinus reducing device under the auspices of the COSIRA-II Investigational Device Exemption study launched earlier this year. The randomized trial is designed to enroll about 380 patients at up to 50 sites — including Cleveland Clinic —to investigate the safety and efficacy of the percutaneously delivered Neovasc Reducer™. The study is the largest sham-controlled device trial ever undertaken for coronary artery disease.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The trial launch follows a period in which the investigational device was available in the U.S. solely on a compassionate-use basis, during which Cleveland Clinic implanted it in eight patients. “We have since begun enrolling patients in the COSIRA-II trial, with all subsequent implants performed according to trial protocol,” says interventional cardiologist Jaikirshan Khatri, MD, who is leading the study at Cleveland Clinic.”
The first COSIRA study, reported in 2015 in the New England Journal of Medicine, found that the coronary sinus reducing device led to significantly improved symptoms and quality of life. The phase 2 trial enrolled 140 patients with Canadian Cardiovascular Society class III or IV angina and myocardial ischemia who were not candidates for revascularization. Patients were randomized to either implantation of the device or a sham procedure. At six months, 71% of patients in the treatment group improved by at least one angina class and 35% improved by at least two classes. These rates were statistically significantly higher than rates in the control group (42% and 15%, respectively).
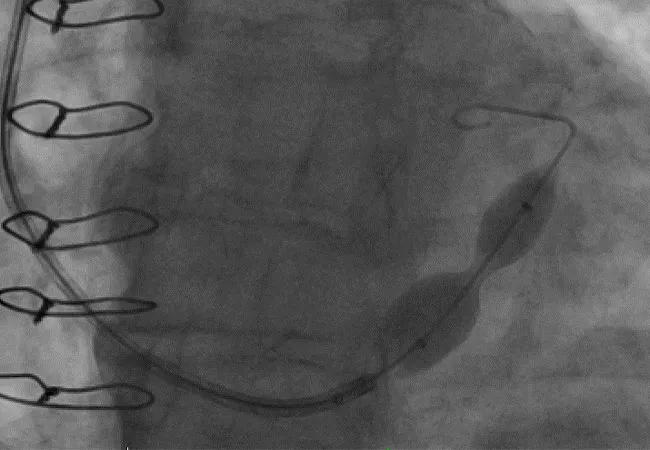
The stainless-steel device is implanted percutaneously in the coronary sinus, where it expands into an hourglass shape, leading to local flow disruption with only the narrow central orifice of the device available for blood flow (Figures 1 and 2). The resultant pressure gradient forces blood to flow from the less ischemic epicardium to the more ischemic endocardium, thereby relieving angina.
Advertisement
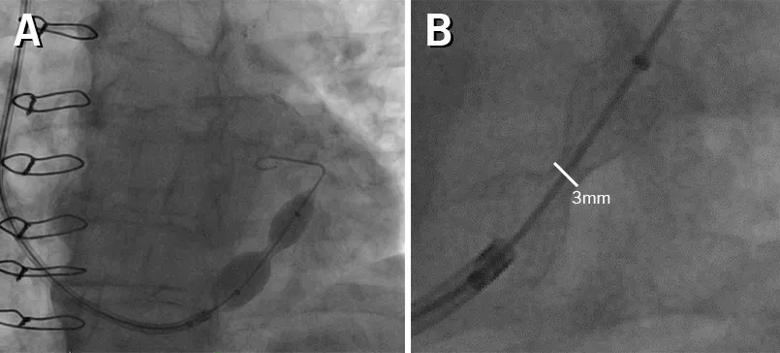
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/067782c3-c5d0-444c-8352-9693c1645400/21-HVI-2340465-Inset1-805x364-1_jpg)
Figure 1. The coronary sinus reducer is an hourglass-shaped, balloon-expandable stent (A) designed to constrain the diameter of the coronary sinus to 3 mm after endothelialization (B).
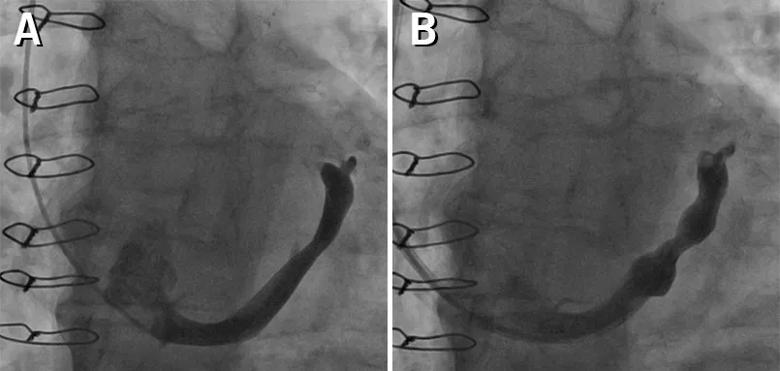
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f215b6f5-e132-42df-ae10-1b22edf60240/21-HVI-2340465-Inset2-805x383-1_jpg)
Figure 2. Angiogram of the coronary sinus reducer at baseline (A) and immediately after deployment (B).
According to Dr. Khatri, Cleveland Clinic’s initial experience with the device in the eight compassionate-use cases showed it to be surprisingly easy to implant, even the first time.
“Although we had never done it before, we implanted all of our initial cases in a total of just a few hours,” he reports. “The patients needed essentially no recovery and went home the same day without complications.”
He notes that clinical outcomes of these cases are not yet fully available, as improvement typically takes about three months to manifest.
The double-blind COSIRA-II investigation is randomizing patients in a 1:1 ratio to either the device or a sham procedure. The primary endpoint is change in exercise tolerance testing time from baseline to six-month follow-up, as measured by a modified Bruce protocol. Eligibility criteria are similar to those for the earlier COSIRA trial, and participants must be ambulatory. After one year, participants in the sham group will be offered the device.
COSIRA-II is enrolling patients with ischemic angina. However, many patients with severe angina have microvascular disease that is not detectable by angiography. These so-called ANOCA or INOCA (Angina or Ischemia and NO Coronary Artery disease) patients may also possibly benefit from the device, especially as stent placement is not an option when there is no blockage to address, notes Cleveland Clinic interventional cardiologist Rishi Puri, MD, PhD.
Advertisement
“This population with severe refractory angina and no evidence of coronary artery disease will be the next frontier for this device,” Dr. Puri predicts. “After COSIRA-II, I expect we’ll see a clinical trial for them.”
The Neovasc Reducer system has been available since 2015 in Europe, where safety but not efficacy must be proven before medical device approval. It is also marketed in Israel and Saudi Arabia. The U.S. FDA granted the product breakthrough device designation in October 2018 but has yet to approve it for commercial use.
“I see a handful of patients each week with severe angina whose lives might be changed by this device,” Dr. Khatri says, which he notes was more than could be accommodated by the previous compassionate-use program.
He and Dr. Puri believe the device will prove safe and efficacious, as suggested by the thousands of patients who have received it in other countries. They expect that with FDA approval, the device could become a game-changer in coronary artery disease management.
“If the coronary sinus reducing device is approved for use in the U.S., it will become an important treatment choice for patients with severe angina who are not candidates for conventional revascularization,” says Dr. Khatri. “Patients with uncontrolled angina are seen in almost every practice. We want to get the word out that there’s an opportunity for appropriate candidates to get this device through COSIRA-II.”
Advertisement
Advertisement

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients

Multimodal evaluations reveal more anatomic details to inform treatment

Insights on ex vivo lung perfusion, dual-organ transplant, cardiac comorbidities and more