Investigational pulsed-field ablation system also yielded procedural efficiencies
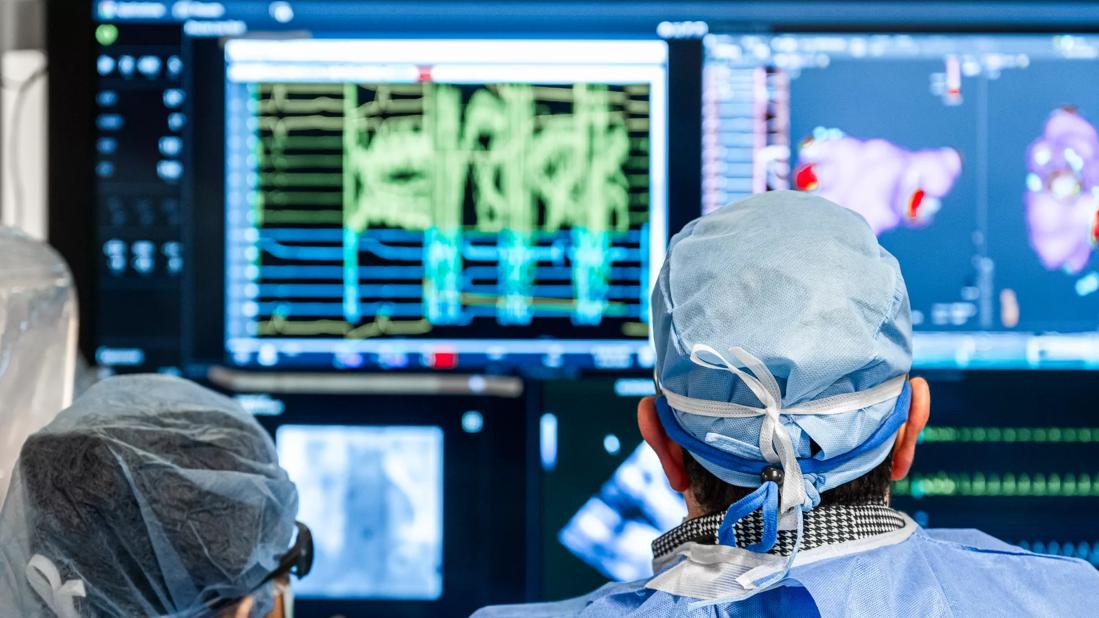
An investigational large-tip lattice catheter combining pulsed-field and radiofrequency ablation capabilities was noninferior to conventional radiofrequency ablation in both safety and effectiveness among patient with persistent atrial fibrillation (AF), found the SPHERE Per-AF trial.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The multicenter randomized study — presented as a late-breaking clinical trial at Heart Rhythm 2024 and simultaneously published online in Nature Medicine — also found the investigational dual-energy catheter to be superior to conventional radiofrequency ablation in measures of procedural efficiency.
“Results of this trial demonstrate highly effective and safe atrial fibrillation ablation with improved procedural efficiency using a novel, multipurpose catheter for high-density mapping and dual-energy ablation delivery,” says study co-author Tyler Taigen, MD, Director of Quality and Associate Section Head of Electrophysiology and Pacing at Cleveland Clinic. He served as site principal investigator of the study at Cleveland Clinic, one of the highest-enrolling sites in the trial.
While catheter-based ablation is an effective and safe treatment for patients with paroxysmal AF, conventional radiofrequency catheter ablation has shown significant limitations in the treatment of persistent AF, with historical one-year success rates of 45% to 62% in clinical trials.
These suboptimal outcomes in the setting of persistent AF are due in part to the technical difficulty of creating contiguous lesions with radiofrequency catheter-based ablation, resulting in conduction gaps and AF recurrence. Additionally, conventional ablation in this setting carries risk of atrio-esophageal fistula, phrenic nerve paralysis and narrowing of the pulmonary vein. Moreover, conventional ablation requires the use of separate catheters for mapping and ablation, increasing procedural complexity and opportunities for complications.
Advertisement
Such shortcomings have led to the development of pulsed-field ablation (PFA) technologies in recent years. PFA is a nonthermal ablation method involving electroporation, or the delivery of rapid, high-voltage pulsed electrical fields to tissue, causing cell membranes to become permeable. In the setting of cardiac ablation, electroporation is performed at an intensity that yields irreversible effects, leading to cell death. The strength of the electrical application can be carefully titrated to destroy only cardiomyocytes and not surrounding tissues, including the esophagus, pulmonary vein and phrenic nerve.
“In contrast to the indiscriminate risk of collateral damage inherent in using thermal energy sources, irreversible electroporation allows operators to focus ablation on cells implicated in atrial or ventricular arrhythmias,” explains Oussama Wazni, MD, MBA, Section Head of Cardiac Electrophysiology and Pacing at Cleveland Clinic.
The SPHERE Per-AF trial (NCT05120193) assessed one particular PFA technology — the Sphere-9™ catheter and Affera™ Mapping and Ablation System (Medtronic) — relative to control therapy with a radiofrequency ablation catheter paired with a multi-electrode mapping catheter and electroanatomical mapping system. The PFA system uses a single dual-energy lattice-tip catheter to deliver either radiofrequency or pulsed-field energy. The catheter has a wider footprint than conventional radiofrequency ablation catheters, allowing creation of wider lesions with the aim of facilitating more-contiguous ablation lines.
Advertisement
“Preclinical and early human studies showed that this novel PFA system generated atrial myocardial lesions and achieved pulmonary vein isolation more efficiently than conventional catheters, with longer durability and without thermal injury to neighboring anatomy,” Dr. Taigen notes. “SPHERE Per-AF aimed to evaluate whether these effects could be replicated in a large randomized trial.”
The study enrolled adults with symptomatic persistent AF refractory to antiarrhythmic drug therapy. An initial “roll-in” phase was conducted involving 37 patients — up to two per site — to allow operators to familiarize themselves with the investigational system. Following that, 432 patients were randomized in a single-blind manner to ablation with either the investigational system or the control system.
Among patients treated with the investigational system, the same lattice-tip catheter was used to create an electroanatomical map of the left atrium and to perform ablation using either radiofrequency or pulsed-field energies. Operators were directed to use pulsed-field energy on the posterior wall, around the left inferior pulmonary vein and close to the phrenic nerve, but they had discretion to use either energy type in all other areas.
Patients were followed for one year. After a three-month blanking period, transtelephonic ECG monitoring was required at least monthly (additional monitoring was triggered by symptoms). Twenty-four-hour Holter monitoring was done at six and 12 months, and 12-lead ECG testing was conducted at three, six and 12 months.
Advertisement
The trial was designed to assess for noninferiority of the investigational system relative to control in terms of effectiveness and safety. The primary effectiveness endpoint was freedom from a composite of treatment failures, including acute procedural failure and repeat ablation at any time as well as arrhythmia recurrence, antiarrhythmic drug initiation/escalation or cardioversion after the three-month blanking period. The primary safety endpoint was freedom from a composite of serious procedure- or device-related adverse events.
Twelve patients dropped out before receiving treatment, leaving 420 patients for the study analysis (212 in the investigational arm, 208 in the control arm). Patients were treated across 20 centers by 40 operators. Operators treated a mean (± SD) of 6 ± 7 patients with the investigational system.
The two study arms were well balanced in terms of baseline clinical and demographic characteristics. Rates of adherence to follow-up were high across the cohort — 96.6% for follow-up visits, 84.1% for Holter monitoring, 85.2% for 12-lead ECG and 92.2% for transtelephonic monitoring — and were virtually identical between the study arms.
The primary endpoint of freedom from treatment failure was achieved by 73.8% of patients in the investigational arm and 65.8% of those in the control arm (P < .0001 for noninferiority). Prespecified superiority testing failed to show superiority of the investigational arm for the primary effectiveness endpoint.
Serious procedure- or device-related safety events occurred in three patients in the investigational arm versus two patients in the control arm (P < .0001 for noninferiority). In all five cases, the event was hospitalization within seven days of ablation. Causes of hospitalization in the investigational arm were pulmonary edema related to hypertensive urgency, exacerbation of chronic obstructive pulmonary disease and hemoptysis; both hospitalizations in the control arm were due to pulmonary edema. There were no reports of atrio-esophageal fistula, pulmonary vein stenosis, tamponade or permanent phrenic nerve paralysis.
Advertisement
Prespecified superiority analysis revealed greater procedural efficiencies in the investigational arm compared with the control arm, including shorter total energy application time, shorter transpired ablation time and shorter procedural time (P < .0001 for all). Additionally, fluoroscopy use and fluid delivery from the ablation catheter were both lower in the investigational arm than in the control arm.
“These findings indicate that this all-in-one mapping and dual-energy catheter is at least as effective and safe as the conventional standard of care for persistent atrial fibrillation, and it appears to offer several procedural efficiencies,” Dr. Taigen notes.
In their study publication, he and his co-authors identify a number of potential benefits of the investigational PFA system, including the following:
“Although further large studies are needed to fully understand the impact of this dual-energy lattice catheter on broader populations with atrial fibrillation, these results demonstrate the promise of this approach to better address the challenges of persistent AF,” Dr. Taigen concludes.
“These positive results from SPHERE Per-AF add to findings from other recent clinical trials in support of other pulsed-field ablation platforms for treating atrial fibrillation,” Dr. Wazni adds. “These studies collectively tell us that pulsed-field ablation holds great promise in making safer ablation available to more patients.”
The study was funded by Medtronic, the manufacturer of the investigational PFA system.
Advertisement

LAA closure may be compelling option in atrial fibrillation ablation patients at high risk of both stroke and bleeding

UK experts compare and contrast the latest recommendations

While results were negative for metformin, lifestyle counseling showed surprising promise
Concomitant AF ablation and LAA occlusion strongly endorsed during elective heart surgery
ACC/AHA panel also upgrades catheter ablation recommendations
For the first time, risk is shown after accounting for underlying contributions of pulmonary disease

Retrospective analysis finds “hypoxic and sleepy” subtype to confer greatest risk
Network proximity and EHR analyses identify diabetes drug as a top candidate for risk reduction