A review of expanding options that hold new promise
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5b61a45e-cf59-411e-92cf-12885c64b300/RHE_5631008_03-06-25_1005_AMO)
Dr. Littlejohn with patient
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The heterogeneity of lupus, both clinically and immunologically, makes disease management and drug development a challenge. For decades, only a handful of FDA-approved drugs existed for this disease, including antimalarials, corticosteroids, aspirin and cyclophosphamide.
By 2020, however, we began seeing a surge in drug development, including:
• Belimumab, a human monoclonal antibody that binds to soluble B lymphocyte stimulator (BLyS) to treat systemic lupus and lupus nephritis.
• Voclosporin, a calcineurin inhibitor approved for lupus nephritis.
• Anifrolumab, a human monoclonal antibody that binds to the type I interferon receptor and is indicated for treatment of systemic lupus.
These drugs have led a long line of therapies that are now emerging for treatment of systemic lupus erythematosus.
The pathogenesis of lupus is complex. It involves pathways starting from exposure of the immune system to autoantigens, presentation of autoantigens to self-reactive T cells, the interactions of T and B cells, and the production of pathogenic autoantibodies by plasma cells. Autoantibodies scavenge autoantigens, producing immune complexes, which interact with plasmacytoid dendritic cells and induce type I interferon. Type I Interferons are heavily involved in pro-inflammatory pathways, including production of pro-B cell survival cytokines (APRIL, BLyS), pathogenic activation of T cells, and signal transduction using the Janus kinase/signal transducer and activation of transcription pathway.
The pathogenic pathways involved in the perpetuation of chronic inflammation in lupus present myriad therapeutic targets, many of which are being explored in phase 1, 2 and 3 trials.
Advertisement
One noteworthy emerging therapy is obinutuzumab, a humanized anti-CD20 monoclonal antibody. This is distinct from other anti-CD20 antibodies (such as rituximab and ocrelizumab) in that it provides greater B-cell cytotoxicity and depletion. NOBILITY, a phase 2 trial using obinutuzumab with mycophenolate mofetil for lupus nephritis class III-IV, met primary endpoint of complete renal response at week 52. Further improvement was evident by week 104.1
Obinutuzumab resulted in greater improvements from baseline in C3, C4 and anti-dsDNA antibodies at weeks 4 through 104, and urine protein-creatinine ratio at weeks 52 through 104. We anticipate positive results from the phase 3 trial ALLEGORY, which is ongoing.
Litifilimab (BIIB059) is a humanized IgG1 monoclonal antibody targeting blood dendritic cell antigen 2. Phase 2 trials have demonstrated this drug to be effective in both cutaneous lupus and systemic lupus as an add-on to standard-of-care therapy. It has met endpoints of decrease in Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) score at week 16 (N = 132) and decrease in number of active joints at week 24 (N = 102).2,3
Deucravacitinib is an oral selective inhibitor of the intracellular signaling kinase, tyrosine kinase
2. Phase 2 trial patients were on background therapy with at least one antimalarial or immunosuppressant drug and could receive up to 30 mg/day of glucocorticoids. Patients treated with deucravacitinib 3 mg twice daily or 6 mg twice daily met the primary outcome of SLE Responder Index (SRI-4) response versus placebo at week 32. Patients treated with deucravacitinib 3 mg twice daily met all secondary end points, including SRI-4, British Isles Lupus Assessment Group-based Composite Lupus Assessment, Lupus Low Disease Activity State, CLASI-50 response, and reduction in active joint count at week 48.4
Advertisement
Ianalumab is an anti-B-cell activating factor receptor fully human IgG1 monoclonal antibody engineered for direct antibody-dependent cellular cytotoxicity-mediated B-cell depletion. In an ongoing phase 3 study,5 406 patients were given monthly or quarterly subcutaneous injection on top of standard-of-care treatment. Primary outcome is SRI-4 response, defined as Systemic Lupus Erythematosus Disease Activity Index –2000 (SLEDAI-2K) reduction from baseline of ≥ 4 points, no worsening in British Isles Lupus Assessment Group –2004, and no worsening in Physician Global Assessment of Disease Activity.
Chimeric antigen receptor (CAR) T cell therapy is the beginning phases as an emerging therapy in rheumatology. CAR T therapy has been used in the oncology space to treat conditions such as B cell non-Hodgkin lymphoma, B cell acute lymphoblastic leukemia and multiple myeloma. CAR T therapy is considered a “living drug,” in which chimeric antigen receptors (also known as chimeric immunoreceptors, chimeric T cell receptors, or artificial T cell receptors) are engineered to give T cells the ability to target a specific antigen. The receptors are considered chimeric because they combine both antigen-binding and T-cell-activating functions into a single receptor. First, a patient’s leukocytes are obtained via leukapheresis, and CD4- and CD8-positive T cells are isolated. T cells then are transfected with the necessary coding DNA to produce a new receptor capable of recognizing a target antigen — in this case, CD19. Once binding to this antigen is achieved, the innate signaling can turn on machinery to destroy the recognized cell.
Advertisement
A recent study of the use of anti-CD19 CAR T therapy for five patients with systemic lupus erythematosus and lupus nephritis yielded exciting results. All patients had SLE according to the EULAR/ACR 2019 criteria, with signs of active organ involvement, including kidney involvement (WHO III or IV). They had failed to respond to multiple immunomodulatory therapies, including pulse dose glucocorticoids, belimumab, azathioprine, mycophenolate mofetil, rituximab and cyclophosphamide. Results were astounding. After three months, all five patients had achieved drug-free remission with decrease in the SLEDAI-2K scores. They also had resolution of proteinuria, improved complement levels, and decreased anti-dsDNA levels.6 These results were maintained over a long-term follow-up, and B cell reconstitution was evident at 150 days. Long-term follow-up in larger SLE cohorts is needed, and these studies are underway.
The therapies discussed are not exhaustive of the potential therapeutic targets or ongoing clinical trials. A recent systematic review of targeted therapies in clinical development yielded a total of 92 (58 biological DMARDs and 34 targeted synthetic DMARDs) in a total of 203 clinical trials.7
It is an exciting time in the systemic lupus space, and we look forward to more treatment options to offer our patients who suffer from this devastating disease.
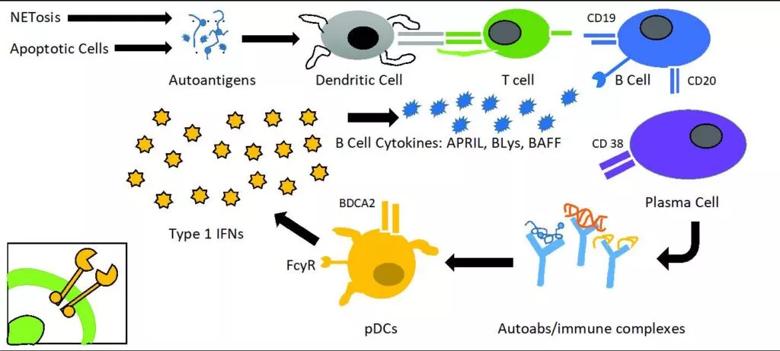
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/81539570-c99a-4d2c-bfc9-c9a4e6f5b5a5/littlejohn-inset-1024x461_jpg)
Illustration adapted from Allen ME, Rus V, Szeto GL. Leveraging Heterogeneity in Systemic Lupus Erythematosus for New Therapies. Trends Mol Med. 2021 Feb;27(2):152-171.
Advertisement
Advertisement
CAR T-cell therapy may offer reason for optimism that those with SLE can experience improvement in quality of life.
Advancements lead to a new trial involving autoimmune disease
Disease associated with higher risks for maternal and fetal morbidities
Benefits of neoadjuvant immunotherapy reflect emerging standard of care
Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies
Why Cleveland Clinic is launching its cardioimmunology center
Collaborative patient care, advanced imaging techniques support safer immunotherapy management
Pembrolizumab does not improve outcomes, but immunotherapy may still offer benefit