Patients receive specialized inpatient and outpatient care for cellular, gene and immune cell-engaging therapies
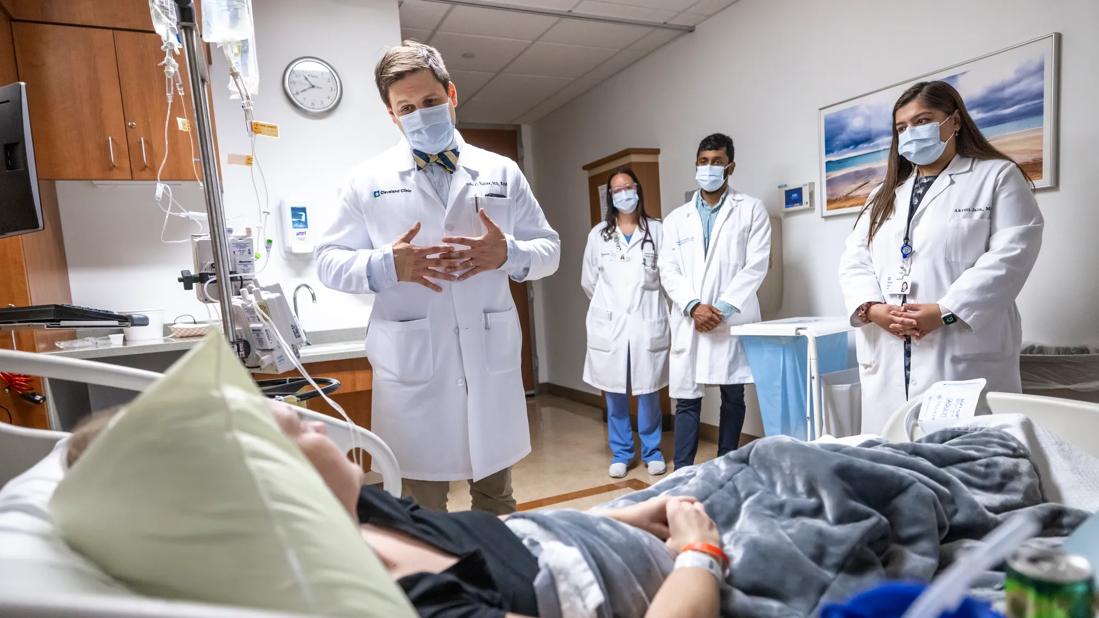
There is a burgeoning demand for treatments such as CAR T-cell, tumor-infiltrating lymphocyte (TIL) and bispecific antibody therapies that leverage the patient’s own immune system to kill cancer cells. These treatments have a unique set of toxicities and require specialized care. To address this growing need, Cleveland Clinic Cancer Institute developed the new inpatient Cellular and Immunotherapy Service (CITS), where patients are treated by a team with specialized training in these emerging therapies.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
This multidisciplinary inpatient service was created to enhance the level of care for patients receiving cellular and immunotherapies with plans to transition some treatments to outpatient settings in the future. Cleveland Clinic Cancer Institute patients receiving any of the below immune effector cell (IEC) and/or immune cell-engaging therapies, either as standard of care or through a clinical trial, are admitted to the Cellular and Immunotherapy Service for treatment.
Initially, most patients in the service will be those with existing FDA-approved therapies including lymphoma, myeloma, melanoma and lung cancer melanomas, and lung cancer. With the new approval of a bispecific immune cell-engaging agent for treating lung cancer, demand for this care is expected to be high. In the future, the hope is to offer cellular therapy treatment to patients with non-malignant conditions, such as multiple sclerosis and lupus, based on the results of ongoing clinical trials at Cleveland Clinic under the leadership of Paolo Caimi, MD, and the Cellular Therapy Research Team.
“The growing patient volumes as well as the specialized care needs necessitated a service focused on these specific treatments approaches,” says Inpatient Director of CITS John Molina, MD, EdM. “As more immunotherapy is moving up earlier in the treatment sequence, a growing number of patients will be receiving these treatments. We set out to design a service and capacity to support more patients.”
Advertisement
In 2024, Cleveland Clinic Cancer Institute is expected to treat 150 patients with CAR T-cell therapy, 87 with bispecific monoclonal antibodies, as well as a handful of patients with TIL, oncolytic virus and gene therapy. The demand for these therapies is expected to more than double by 2028. As the use of these medications expands through research portfolios and standard of care, demand is high for clinicians with expertise in these newer treatment approaches.
Patients receiving an IEC product within the service receiving any cellular products will be treated on an inpatient unit accredited by the Foundation for the Accreditation of Cellular Therapy (FACT). Co-founded by the International Society for Cell and Gene Therapy and the American Society for Transplantation and Cellular Therapy, FACT created rigorous standards for hospitals delivering cell therapy. Building on this expertise, as well as its clinicians’ years of experience treating patients with these therapies, the clinic has been:
Advertisement
As with other parts of the Cancer Institute, CITS has its own tumor board to review complex cases and challenging toxicities. The CITS team also plans to partner with community providers in care delivery. “Community hospitals may not be able to offer the initial cycles of these therapies, but we can help them facilitate follow-up care where appropriate,” says Dr. Molina.
As more cell and gene therapies are being offered in an outpatient setting, the center has built the experience to support this level of care. “For some therapies like certain CAR T-cell treatments, patients can receive the initial lymphodepleting chemotherapy in the outpatient setting for the days leading up to the cell therapy infusion. This buys them more time outside of the hospital and shortens the hospital stay for patients,” says Dr. Molina.
With this infrastructure in place, center leaders anticipate opening more cellular and immunotherapy research studies. In addition to the CTRT program lead by Dr. Caimi, other oncology disease groups looking to offer these cutting-edge IEC therapies to their patients will have the support of CITS for the delivery and toxicity management of these novel agents.
“Trial sponsors appreciate that we have dedicated space and infrastructure from a research standpoint,” says Dr. Molina. “Other institutes can bring their research to help conduct studies both inside and outside of oncology. Many companies are also looking to research these types of immunotherapy products for non-hematologic diseases.”
Advertisement
Advertisement

Collaborative patient care, advanced imaging techniques support safer immunotherapy management

Highly personalized treatment shrinks tumors resistant to immunotherapy

Cleveland Clinic Cancer Institute among select group of centers to administer highly personalized treatment

Researchers develop first-of-its-kind neoantigen atlas to better understand immunotherapy resistance
Clinicians highlight the need to educate patients about warning signs

Immune toxicity remains a diagnosis of exclusion, and multidisciplinary collaboration remains the cornerstone for early diagnosis and treatment.

Benefits of neoadjuvant immunotherapy reflect emerging standard of care

Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies