Research holds hope for restoring some cognitive abilities
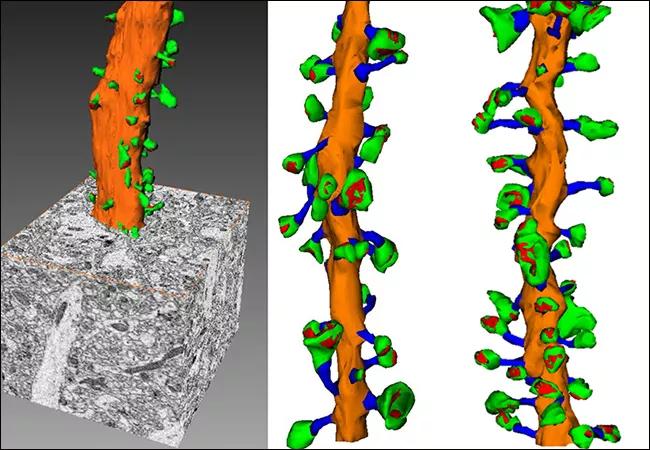
Cleveland Clinic researchers have discovered for the first time that faulty connections between neurons causes fragile X syndrome (FXS), the leading inherited cause of intellectual disability and autism spectrum disorders. FXS is caused by mutations that silence the FMR1 gene (fragile X mental retardation 1).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The researchers, led by Bruce Trapp, PhD, Chair of Neurosciences in the Lerner Research Institute, used the extremely sensitive 3-D electron microscopy to visualize and characterize neurons in the brains of healthy mice versus mice with FXS. They focused their research in the hippocampus. According to Dr. Trapp, 3-D electron microscopy provides far more sensitivity than traditional light microscopy, allowing nanoscale visualization.
With this technology, Dr. Trapp’s team closely examined the size and shape of dendritic spines, which are branch-like extensions of neurons that receive signals from other nerve cells. They discovered that the mice with FXS (FMR1 knockout mice) have more dendritic spines than normal mice. There also appeared to be fewer mature dendritic spines.
The researchers went on to show that this change in FXS mice was caused by disrupted “housekeeping” of nerve cells that occurs in early development — called synaptic pruning. “Synaptic pruning is essential for removing faulty or immature nerve cell connections in the young brain,” Dr. Trapp says. “When this process malfunctions, it can cause an overabundance of defective neurons that are not able to receive signals properly.”
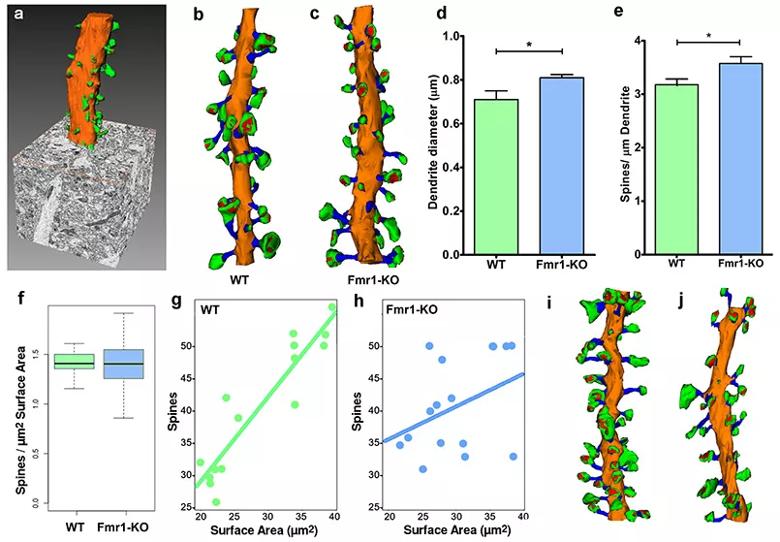
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/0ae4b5f3-2c4d-4b31-90dd-bacb1bacc07e/805x-Inset-1-Autism_jpg)
Figure 1: Three-dimensional EM analysis of secondary dendrites from P60 WT and Fmr1-KO CA1 hippocampal neurons. (a) Secondary dendrites were reconstructed from stacks of up to five hundred 75 nm-thick sections collected at 7 nm/pixel resolution. (b-c) Dendritic shafts and dendritic spines from WT (b, 10 µm) and Fmr1-KO (c, 10 µm) mice were analyzed. (d) The diameter of secondary dendrites was significantly increased in Fmr1-KO mice. (e) Dendritic spine density was increased in Fmr1-KO mice. (f) The mean number of spines per dendritic surface area was similar in WT and Fmr1-KO mice, but the variance was significantly higher in the Fmr1-KO group (F-test, p<0.04). (g,h) Spine numbers linearly correlate with dendrite surface area in WT mice (g; r2=0.85). Spine number does not correlate with dendritic surface area in Fmr1-KO mice (h; r2=0.08). Comparison of two Fmr1-KO dendrites illustrates the high variability in spine density in dendrites of similar diameter (i,j).
Advertisement
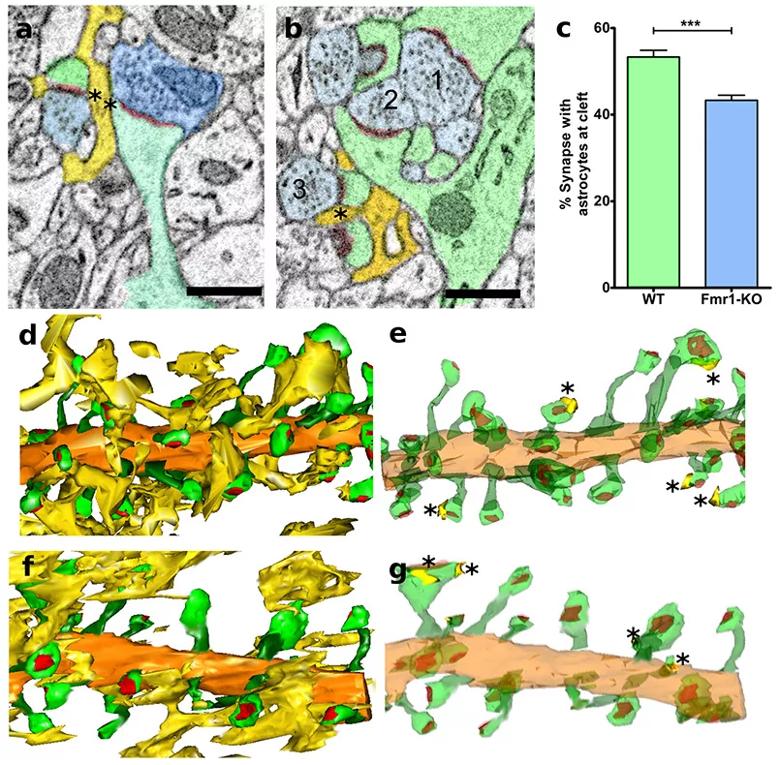
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/dd450372-2032-4a86-b56c-76c05fb47edb/805x-Inset-2-Autism_jpg)
Figure 2: Astrocyte participation in the tripartite synapse is decreased in Fmr1-KO mice. (a,b) Single slice electron micrographs showing a synapse with (a, b; 1-2) and without (b; 3) an astrocyte process opposing the synaptic cleft (asterisks = apposition; yellow = astrocyte process; blue = presynaptic terminal; green = dendritic spine; red = PSD). (c) Synaptic clefts with astrocyte processes were significantly greater in WT than in Fmr1-KO mice. (d-g) Three-dimensional reconstructions of astrocyte processes (yellow), dendritic spines (green), and PSDs (red) in WT mice (d, e) and Fmr1-KO (f, g) mice. d-g Reconstruction of the tripartite synapse in WT (d, e) and Fmr1-KO (f, g) mice; e and g, 3D reconstructions of astrocyte-synapse associations; d and f, all astrocyte processes, e.g. astrocyte contact zones (WT: N = 3 animals, 3 dendrites, 121 spines; Fmr1-KO: N = 3 animals, 3 dendrites, 101 spines; ***p<0.001), Scale bars (a, b) = 0.5 µm
This groundbreaking research suggests that enhancing synaptic pruning to reduce the number of faulty or immature neuronal connections may help restore some cognitive abilities in patients with autism spectrum disorders. Additionally, further research may explore restoring the function of the silenced FMR1 gene.
According to the authors, one percent of the world’s population are affected by an autism spectrum disorder, and one in 68 newborns in the United States, demonstrating the urgent clinical need for better neurological and genetic understanding of the diseases.
Advertisement
Safar Jawaid, PhD, is first author on the paper, which was published online in the December 23 issue of GLIA. Dr. Trapp is an internationally renowned neuroscientist. In 2017 he received the prestigious Outstanding Investigator award from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health.
The research was supported by a grant from the National Institute of Mental Health, NIH.
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity

Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time

Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology