ENRICH trial marks a likely new era in ICH management
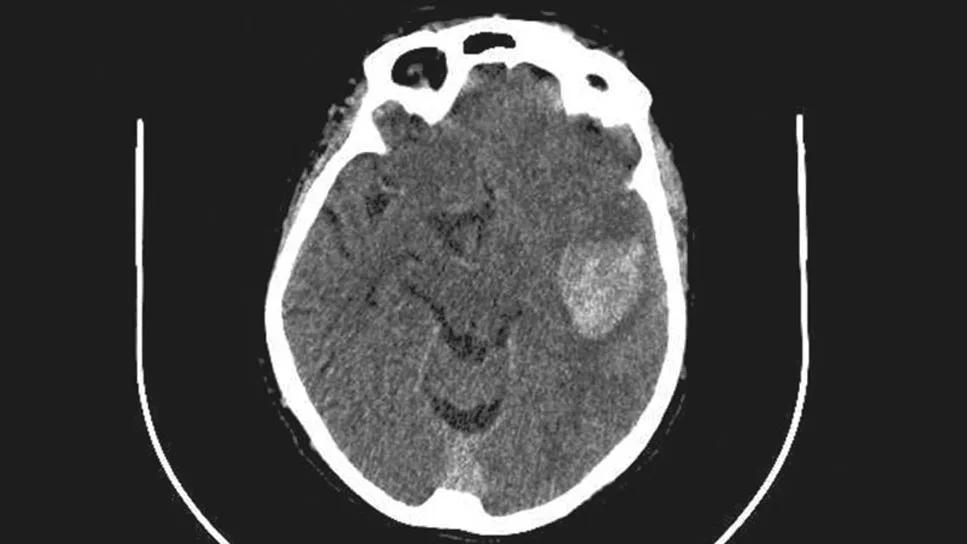
For the first time, a randomized trial has shown that surgical intervention is superior to standard medical management for treatment of supratentorial intracerebral hemorrhage (ICH). That’s the central takeaway from the multicenter ENRICH trial evaluating ICH evacuation with a minimally invasive parafascicular surgery approach. The study was published in the New England Journal of Medicine.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“This is a pivotal moment in the treatment of intracerebral hemorrhage that’s analogous to the long-awaited validation of endovascular therapy for acute ischemic stroke back in 2015,” says Cleveland Clinic vascular neurosurgeon Mark Bain, MD, MS, who served on the ENRICH trial’s planning committee is a study co-author. “I see ENRICH as the beginning of an era of minimally invasive surgical treatment of intracerebral hemorrhage. I expect a large uptick in cases worldwide that are treated surgically in the wake of these results.”
ICH has long been a devastating and therapeutically elusive cerebrovascular disease, accounting for some 10% to 15% of all strokes and a disproportionate share of stroke-associated morbidity and mortality. Treatment guidelines recommend medical management involving aggressive treatment of coagulopathy and blood pressure along with prevention of secondary brain injury and control of intracranial pressure. However, medical management is inadequate in many cases, as up to 40% of patients die within 30 days of ICH and about 80% of those who survive are left with significant disability.
To date, surgical management has not clearly improved outcomes. Randomized trials of surgery for ICH — including the STICH and STICH II trials — have failed to demonstrate functional or cognitive improvements over medical management. Surgery is believed to have been limited in these studies by delayed intervention, heterogeneity in surgical approach and/or insufficient clot removal.
Advertisement
The STICH trials used open craniotomy in a large majority of cases, leading to speculation that the trauma created by invasive surgery may negate any benefit of hematoma evacuation. This spurred interest in minimally invasive approaches, most notably with the MISTIE procedure involving minimally invasive placement of a catheter to initiate hematoma aspiration followed by intermittent dosing of a thrombolytic to achieve clot lysis. Unfortunately, the MISTIE III trial found that this approach yielded no significant advantage over medical management in functional outcomes, although the results suggested greater benefit with greater hematoma removal and lower end-of-treatment clot volume.
The ENRICH trial evaluated a different minimally invasive approach to ICH — namely, minimally invasive parafascicular surgery (MIPS). The procedure uses two commercially available tools — the BrainPath® tubular retractor system and the Myriad NOVUS® tissue removal technology, both from NICO Corporation. BrainPath, which can be inserted using stereotactic targeting after a small craniotomy, is designed to access subcortical spaces with minimal trauma to cortical structures and white matter tracts. Myriad NOVUS is designed for minimally invasive removal of liquefied or cross-linked clots through cutting, suction and blunt dissection.
Because these technologies are approved by the FDA for removal of brain tissue in other settings, they had been tried for ICH evacuation prior to the ENRICH trial. Dr. Bain and Cleveland Clinic colleagues were early adopters of BrainPath for ICH evacuation, publishing a series of cases in Operative Neurosurgery (2017;13:69-76). “A number of institutions have been using it for ICH evacuation for several years now,” says Dr. Bain. “Our use dates back to 2013. Outcomes have been good, but there hadn’t previously been randomized trial data to support this use.”
Advertisement
The ENRICH trial was undertaken to try to change that. Three hundred adults with supratentorial ICH at 37 U.S. centers were randomized 1:1 to either standardized medical management (MM), based on guidelines from the American Heart Association/American Stroke Association, or MIPS using the two NICO devices detailed above. Intervention was initiated within 24 hours of symptom onset. Randomization was stratified by ICH location — i.e., lobar or anterior basal ganglia (ABG).
All participating neurosurgeons completed a training course in standardized application of the MIPS procedure. Of the study’s 300 patients, 26 were enrolled at Cleveland Clinic, the most of any center.
The primary efficacy endpoint was utility-weighted modified Rankin scale score (UWmRS) at 180 days, with a weight of 1.0 for an mRS score of 0 (no symptoms) and a weight of 0 for a score of 5 (severe disability) or 6 (death). “This utility weighting has been used in multiple other studies of cerebrovascular disease,” Dr. Bain notes. “It’s a more patient-centric metric and reflects outcomes with greater precision and nuance than reliance simply on mRS scores or score ranges.”
The trial used a prespecified adaptive design similar to the designs of other recent large cerebrovascular studies, with the intent of reaching the overall study conclusion as soon as possible. The design allowed for enrichment based on ICH location, with results at interim analyses enabling enrollment of patients with a given ICH location to be stopped in favor of those with the other ICH location if the other location were associated with a greater magnitude of benefit from MIPS.
Advertisement
At the study’s second interim analysis (after enrollment of 175 patients), the prespecified stopping criterion was met for the ABG location, which triggered all subsequent enrollments to be done with patients meeting the lobar location criteria. This led to the following breakdown of the study’s 300 patients: 208 (69.3%) had ICH in the lobar location and 92 (30.7%) had ICH in the ABG location. No significant baseline differences were observed between the MIPS and MM groups in age, ICH volume, Glasgow Coma Scale score, National Institutes of Health Stroke Scale score or other assessed demographic and clinical factors.
An mRS score at 180 days was obtained in 286 participants (95.3%): 139 in the MM group (40 ABG and 99 lobar) and 147 in the MIPS group (47 ABG and 100 lobar). On the primary endpoint, the mean UWmRS at 180 days was 0.37 for the MM group and 0.46 for the MIPS group, a difference of 0.084. This yielded a Bayesian posterior probability of superiority of MIPS over MM of 98.1%, which exceeded the prespecified 97.5% threshold for superiority. Ordinal logistic regression analysis showed that the superiority of MIPS emerged early (at discharge) and was consistently maintained at 30, 90 and 180 days.
Analysis by ICH location showed that the superiority of MIPS over MM was apparently attributable to the results in patients with hemorrhage in the lobar location.
Outcomes on the leading safety endpoints significantly favored MIPS over MM: all-cause mortality at 30 days (9.3% vs. 18.0%, respectively) and change in hemorrhage volume from index CT to 24-hour CT (–43.9 mL vs. 4.0 mL). The other safety endpoint — postoperative bleeding related to clinical deterioration — occurred in five MIPS patients (3.3%).
Advertisement
Overall mortality at 180 days — a prespecified secondary endpoint — favored MIPS over MM (20.0% vs. 23.3%), although the difference was not statistically significant. The MIPS group had significantly shorter length of stay in both the intensive care unit (2.8-day difference) and the hospital (3.1-day difference). In the MIPS group, the median extent of hemorrhage evacuation was 87.7% and the mean end-of-treatment hemorrhage volume was 14.9 mL. The goal end-of-treatment volume of ≤ 15 mL was achieved in 72.7% of patients in the MIPS group.
“This is the first clinical trial to demonstrate the functional benefit of surgical clot evacuation in patients with supratentorial ICH presenting within 24 hours,” says Dr. Bain. “It also confirmed the safety of MIPS and its ability to achieve substantial hematoma evacuation. These results validate the approach we have taken for several years now for many patients at Cleveland Clinic, but it represents a paradigm shift for treatment of ICH more broadly. And the protocol development and training behind this trial have helpfully standardized the approach to MIPS to optimize patient outcomes.”
He notes that while superiority for MIPS was demonstrated across the entire cohort, this was driven by the strong positive effect observed for patients with lobar ICH. “The benefit for patients with lobar ICH is clear,” he says. “We need to do further investigation in patients with hemorrhage in the anterior basal ganglia, as that’s a more sensitive location. While those patients can still fare well, we need to select them properly.”
To better guide that patient selection, the ENRICH investigators will closely analyze data from the trial’s ABG cohort, a project that will be coordinated by Cleveland Clinic. Dr. Bain suspects that strategies to improve outcomes in this setting might include choosing patients with smaller hemorrhage volume, using additional imaging modalities to improve patient selection and employing smaller devices. After further analysis of the ABG data from ENRICH, a randomized trial specific to the ABG location — and with a larger ABG cohort — is highly likely, he says.
Meanwhile, Dr. Bain expects that the effects seen in ENRICH are likely to apply to other devices that can be used for MIPS, both in development and already commercially available. “In addition to an increase in ICH cases treated medically, I think these results are going to spur further development of specialty instruments for this procedure with additional refinements,” he says. “As the devices improve and proliferate, the outcomes will only get better.”
The ENRICH trial received funding from NICO Corporation.
Advertisement

Results may refine surgical selection criteria and advance clinical trial design
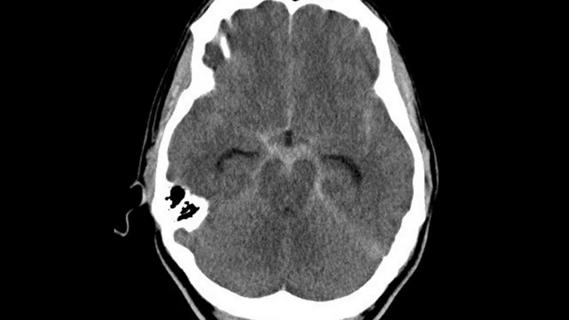
Digital subtraction angiography remains central to assessment of ‘benign’ PMSAH

Quick and aggressive responses to multiple complications have led to remarkable recovery
Case series links catastrophic outcomes to therapy initiation within 48 hours post-procedure
Scientific program chair reflects on what may resonate longest from this year’s neurosurgery conference

An argument for clarifying the nomenclature

An expert talks through the benefits, limits and unresolved questions of an evolving technology

Recommendations on identifying and managing neurodevelopmental and related challenges