What it could mean for the future of ambulatory monitoring for bladder conditions
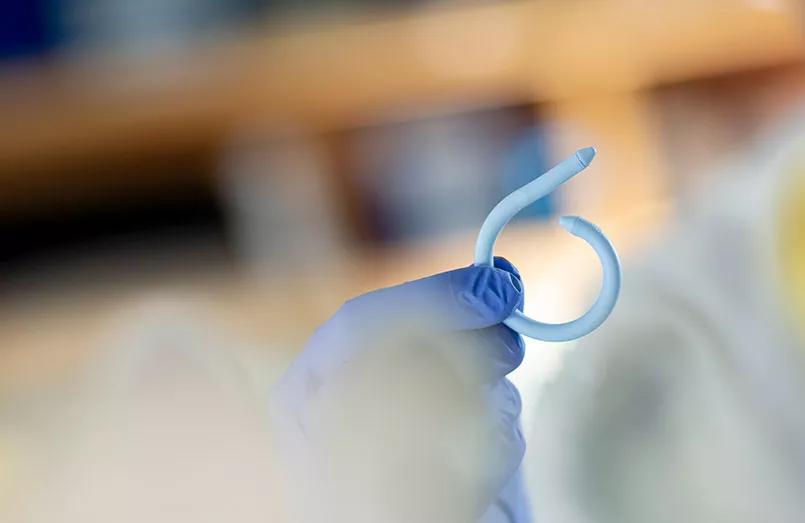
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6892c140-42ab-4adf-a5f9-b43f9cd7067e/805x-inset-Damaser_jpg)
805x-inset-Damaser
The UroMonitor, a wireless, insertable pressure sensor to assist in the diagnosis of urinary incontinence and other bladder disorders, is safe, feasible and well-tolerated in women with refractory overactive bladder (OAB), according to clinical trial data reported in the Journal of Urology.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Margot Damaser, PhD, and colleagues in the Biomedical Engineering Department of Cleveland Clinic Lerner Research Institute have been developing the device for more than a decade. The trial data represents the first step in validating its comparative clinical benefit compared to conventional urodynamic testing.
The innovation was developed in response to conventional urodynamics, which have several limitations. Catheter-based testing is painful and embarrassing — patients must force urination in an office setting to replicate the symptoms they experience in their daily lives. And because of the artificial conditions, the simulation is not always an accurate reflection of patients’ experiences, which is problematic for physicians.
Dr. Damaser comments, “Our vision is to take the monitoring out of the clinic and take the catheter out of the monitoring.” Dr. Damaser has been working closely with urologists, including Howard Goldman, MD, staff in the Department of Urology and clinical lead on the study, along with researchers and physician collaborators at Case Western Reserve University and the Louis Stokes Cleveland VA Medical Center.
Initial data from the trial were presented at the 2021 American Urological Association Meeting, earning recognition from colleagues, including the Best Paper Award from the Engineering and Urology Society.
The trial consisted of 11 adult female patients, all with indications for urodynamics for refractory OAB, and began with a baseline assessment using standard multi-channel urodynamics (UDS). After that, the investigators inserted the device transurethrally, as would be done in cystoscopy or catheter placement, with a silk suture attached to one end of the device and taped to the patients’ thigh for easy retrieval from the bladder.
Advertisement
How does it work? The device’s medical silicone-coated sheath that houses the pressure-sensing technology curls into a pigtail shape upon insertion to remain within the bladder. It transmits vesical pressure data at 10 Hz to a device taped to the patients’ abdomens.
Alongside the UroMonitor, the investigators performed a second urodynamics. Once the UDS catheters were removed, leaving only the UroMonitor in place, the patients were encouraged to ambulate and void. The team assessed patient discomfort at every stage using visual-analog pain scales, and overall comfort and safety during testing were also evaluated.
In addition to being easily inserted and extracted in all participants, the device successfully captured 98% (85/87) of voiding and non-voiding urodynamic events, according to the authors. Additionally, low post-void residual volume and low overall pain were noted.
Median ambulatory pain score with the device was rated 0 (0-2). No infections or changes to voiding were noted after the procedure.
“Patients reported minimal discomfort during the insertion phase, but nothing unexpected, and there were no post-procedure complications to report,” says Dr. Goldman, adding that physicians and patients alike were pleased with the experience.
While the results demonstrated with the prototype are encouraging, he emphasizes the feasibility study was designed to assess safety and accuracy—and is not powered to draw conclusions about comparative clinical value over UDS. Currently, the UroMonitor measures bladder pressure but does not include bladder volume or have diagnostic capabilities. Although, plans for future iterations will change the scope of these parameters.
Advertisement
The team has already begun another arm of the trial to test the device in patients with multiple sclerosis. The researchers are hopeful that the device may one day play a therapeutic role for patients with neurogenic etiologies, noting that possible applications include controlled drug delivery and feedback to neuromodulation or electrical stimulation systems. The device may also restore sensation for patients with pelvic floor dysfunction or inform them about when to empty their bladder.
For now, though, the team is pleased to report the feasibility of a catheter-free wireless bladder pressure-sensing device that can be used safely in humans during ambulation and voiding. Extended monitoring times — in the comfort of patients’ homes — could offer a more accurate and patient-centered approach to diagnosing lower urinary tract dysfunction.
Advertisement
Advertisement
The low anterior access approach using the single-port robot is gaining attention within the field
Revolutionizing pediatric urology with a new, less invasive approach
Retrospective study shows SGLT-2 inhibitors may lead to worse urologic outcomes
Study reinforces the benefits of using cystatin C
Cleveland Clinic surgeons describe a new technique for harvesting a rectal mucosa graft
New study sheds light on national trends in urology residency, former CCLCM alum comments
Pediatric urologists lead quality improvement initiative, author systemwide guideline
Fixed-dose single-pill combinations and future therapies