Can a single subretinal injection provide effective treatment?
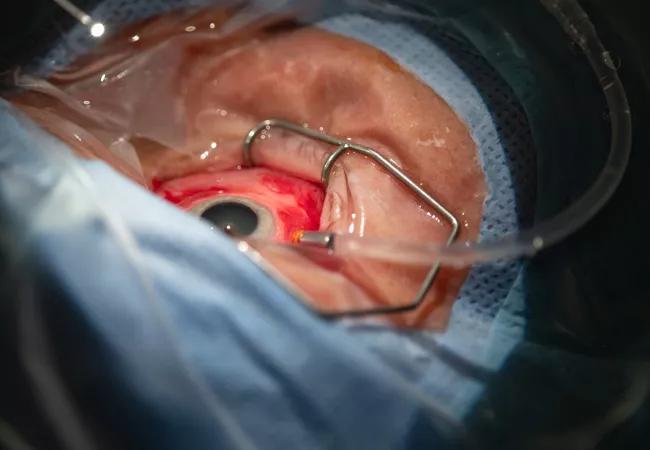
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b1df23cd-4116-4322-a543-200f69a7319e/21-EYE-2531308_Age-Related-Macular_99_Hero_650x450_jpg)
Gene therapy surgery
Recent results of the phase 3 DERBY, OAKS and GATHER1 trials have created excitement about a potential breakthrough therapy for geographic atrophy (GA), the late form of dry age-related macular degeneration (AMD). The trials showed that intravitreal injections of pegcetacoplan or avacincaptad pegol significantly reduced lesion growth, slowing progression of a disease previously thought untreatable.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
One caveat, however, is that injections could be needed monthly or every other month for the rest of a patient’s life. A new effort at Cleveland Clinic Cole Eye Institute and other medical centers is exploring a potential alternative, a one-and-done gene therapy surgery.
“Fifteen years ago, large-scale genome studies identified a link between late-stage AMD and variants in the gene that produces a key complement protein,” says Alex Yuan, MD, PhD, a vitreoretinal surgeon at the Cole Eye Institute. “Separately, Cole Eye Institute researchers Joe Hollyfield, PhD, and John Crabb, PhD, in 2002 and 2010 found an abundance of complement proteins in cadaver eyes of AMD patients compared with controls. Together, these studies and others provided strong evidence that the complement cascade is involved in dry AMD and GA.”
Complement modulation appears to reduce the progression of GA, according to DERBY, OAKS and GATHER1 studies.
“Only with these recent phase 3 studies did enthusiasm start to build in this field,” says Peter K. Kaiser, MD, a vitreoretinal surgeon at the Cole Eye Institute. “With monthly or every-other-month injections we can reduce progression of GA and loss of vision. Thus, finding something more permanent — with gene therapy, where we do the same thing with one treatment — makes a lot of sense.”
In September 2021, interim results of the phase 1/2 FOCUS study indicated that an investigational gene therapy that produced complement factor I showed encouraging early signals. Complement factor I levels remained elevated compared to baseline in most patients (11 out of 13), even more than one year after treatment in some patients. Since complement factor I helps reduce the abnormal activation of the complement cascade, producing it could work to reduce damage to the retina.
Advertisement
Now two additional phase 2 trials, EXPLORE and HORIZON, are studying the effect of the gene therapy administered as a single subretinal injection. The Cole Eye Institute is a study location for both trials.
Dr. Kaiser performed the first gene therapy surgery for GA at the Cole Eye Institute in November. Since then, two more patients have been treated, with more to follow in 2022.
The outpatient surgery was performed in approximately 30 minutes, using an operating microscope and other specialized equipment to deliver a vector — a modified adeno-associated virus — carrying a gene that expresses complement factor I to the retina.
“The goal of treatment is to get the viral vector into the subretinal space, where it will transduce the retinal pigment epithelium to produce complement factor I,” explains Dr. Kaiser. “We perform a standard vitrectomy procedure, and then make one or more localized retinal detachments [blebs of fluid] with a very small delivery instrument attached to a 41-gauge cannula. In the blebs of fluid, we inject the gene therapy. By the next day, the localized retinal blebs have been reabsorbed.”
Each patient receiving this investigational treatment will be followed for two years. Patients then will be invited to join a follow-up study to help researchers further understand the investigational treatment over a longer period of time.
“Patients in these trials already have GA,” says Dr. Yuan, Cleveland Clinic’s principal investigator for the trials. “Our first patient had lesions partially involving the fovea and had begun losing vision. However, the trials also include patients who have lesions completely outside the fovea and have not yet lost central vision. The hope is that this treatment will reduce the risk of GA progression and vision loss.”
Advertisement

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/aa30b979-2281-4204-81c7-e140af5a05d6/age-related-macular-degeneration-1)
The Cole Eye Institute team prepares to perform its first gene therapy surgery for geographic atrophy in November 2021. At center is Alex Yuan, MD, PhD, a principal investigator for the clinical trial.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d175a4dd-1a3b-45b4-949c-20510001c2df/age-related-macular-degeneration-2)
Dawn Williams, RN, prepares the gene therapy vector solution for subretinal injection.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/df267c71-4ede-45c8-a42e-b77428eb65fd/age-related-macular-degeneration-3)
The patient is prepped and draped to begin the procedure.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6632ed7b-1197-4781-8f0c-af6a9cc78bf6/age-related-macular-degeneration-4)
Vitreoretinal surgeon Peter K. Kaiser, MD, uses an operating microscope to magnify the view of the delicate ocular structures.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/8690f08d-293b-4f6d-bd0f-9ce8b8221543/age-related-macular-degeneration-5)
The surgical team creates a subretinal bleb into which the gene therapy product will be delivered.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/48b9e3cb-08d6-4342-9c3b-3fa4c150b782/age-related-macular-degeneration-6)
It requires many hands in a small space to deliver the gene therapy product under the retina.
Gene therapy surgery may offer even more hope for patients with GA, who until recently were told that treatment did not exist.
“Unlike wet AMD, for which we have treatments to preserve and possibly restore vision, GA has been especially frustrating,” says Dr. Yuan. “It can have a devastating effect on patients. Gene therapy surgery may provide a new opportunity to slow this disease.”
Advertisement
Advertisement
Study identifies factors that may predict vision outcomes in diabetic macular edema
Flaps, blebs and other surgical options
Greater central subfield thickness and better visual acuity at baseline are associated with longer time to resolution
Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics
Genetic variants exist irrespective of family history or other contributing factors
CD36 loss-of-function variant accounts for large portion of risk in this population
First-in-human phase 1 trial induced loss of function in gene that codes for ANGPTL3
Paired blood and brain tissue methylation findings raise prospect of noninvasive precision diagnosis