NIH grant bolsters research to refine choice of ablation candidates and targets
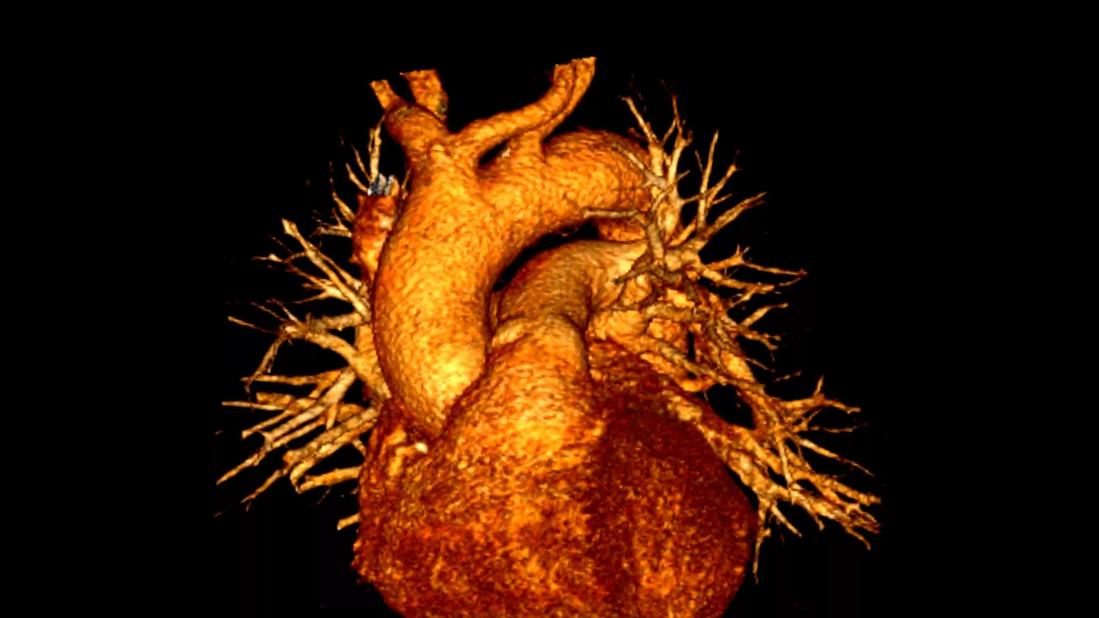
Computer analysis of morphological features from cardiac computed tomography (CT) may one day help better predict patients at risk for post-ablation recurrence of atrial fibrillation (AF) and identify optimal ablation targets.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Now that goal appears closer to realization thanks to a new four-year, $2.95 million National Institutes of Health grant to a consortium led by Cleveland Clinic and Case Western Reserve University researchers. The team is combining radiomics and genomics to conduct investigations with the goal of aiding patient and clinician decision-making around the risk of AF recurrence.
“The expanding development of artificial intelligence methods that can be used to analyze imaging data provides great potential to benefit the field of atrial fibrillation,” says Cleveland Clinic cardiologist Mina Chung, MD, who serves as a co-principal investigator on the project along with John Barnard, PhD, of Cleveland Clinic’s Department of Quantitative Health Sciences and Anant Madabhushi, PhD, of the Case Western Reserve School of Medicine and Case School of Engineering. “We are using novel approaches to develop tools that we anticipate will improve on those currently used for choosing candidates for catheter ablation and determining areas to ablate.”
Predicting which patients will have post-ablation AF recurrence is a long-standing problem. The success rate of catheter ablation is approximately 80% to 90% in paroxysmal AF but can be less than 50% to 60% in persistent AF. There can also be about a 1% to 5% risk of major complications, especially from excessive ablation in patients who require reablation.
Although pulmonary vein isolation is the cornerstone of the procedure, modest success rates have led to additional targeting of areas that have not been well studied. Clinical risk factors, such as persistence of AF and left atrial scarring, can predict worse success rates. Due to the clinical burden of AF and limitations of current therapies, the National Heart, Lung, and Blood Institute has made research on AF a major priority.
Advertisement
Over the past few years, the Cleveland Clinic/Case Western Reserve research group has used contrast-enhanced CT angiograms to capture subtle shape and textural differences in various anatomical areas between patients who had AF recurrence following ablation and those who did not. They identified variations around the left atrial appendage and pulmonary veins that appear to be predictive of AF recurrence.
A “fractal radiomics” technique significantly improved prediction of AF recurrence compared with models using clinical features or atrial volume measurements alone, as the group reported in BMC Medical Imaging (2021 Mar 9;21[1]:45) and Circulation: Arrhythmia and Electrophysiology (2021;14[3]:e0009265).
The investigators also contributed to genome-wide association studies that have identified gene loci and single nucleotide polymorphisms that are associated with AF and post-ablation AF recurrence. Their findings suggest that genes involved in AF pathogenesis can impact anatomic features detectable by imaging.
The locus with the strongest association with AF is near PITX2, a transcription factor expressed early in development that appears to suppress a left atrial sinus node program and is involved with formation of the pulmonary veins, the target of AF ablation.
The NIH grant will enable the team to retrospectively analyze pre-ablation CT scans from more than 2,000 patients who have undergone AF ablation. These will be evaluated morphologically using machine learning techniques with regard to post-ablation outcomes.
Advertisement
Dr. Chung identifies three main goals of the current research:
“Our work is unique in combining artificial intelligence with the use of radiomics and genomics to focus on atrial fibrillation ablation outcomes,” says Dr. Chung.
“While most of our work so far at the Center for Computational Imaging and Personalized Diagnostics (CCIPD) has focused on the use of radiomics and machine learning for predicting outcomes and treatment response for various cancers, this project is an exciting opportunity to validate these approaches for predicting response to treatment for atrial fibrillation,” adds Dr. Madabhushi, the Donnell Institute Professor of Biomedical Engineering and Director of the CCIPD at Case Western Reserve.
Advertisement
“We anticipate that the findings of this research will enable development of personalized analytic tools that can be used in clinical trials to predict targets in patients undergoing atrial fibrillation ablation,” observes Oussama Wazni, MD, Section Head of Electrophysiology at Cleveland Clinic. “The aim is for these tools to eventually help physicians better determine risks and benefits of ablation for their patients.”
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity

Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time

Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology