Findings implicate hydrogen sulfide dysfunction, point to potential treatment strategies
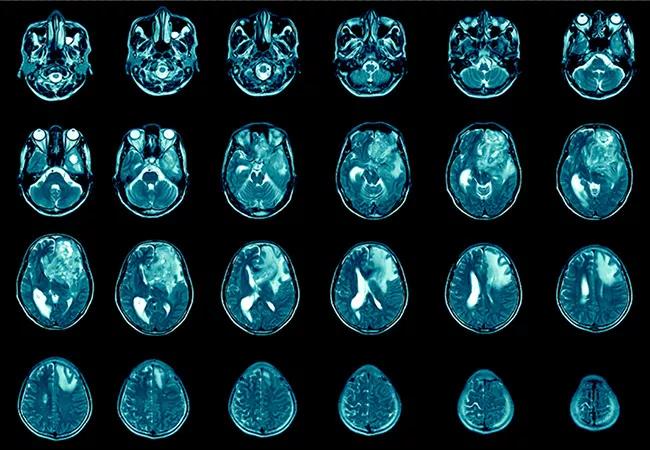
A high-fat diet accelerates and intensifies glioblastoma in preclinical models, according to a recent study by Cleveland Clinic researchers published in the Journal of Clinical Investigation (2021;131[17]:e138276). The findings highlight the need for more research into the possible utility of dietary interventions to reduce the risk and severity of glioblastoma in humans.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Specifically, the study identified hydrogen sulfide as a key player in the diet-glioblastoma link. Findings suggest that increasing the bioavailability or production capacity of hydrogen sulfide in combination with current standard-of-care glioblastoma therapies may improve treatment efficacy.
The need for such improvement in glioblastoma, the most common primary malignant brain tumor, is great.
“Glioblastoma has one of the most aggressive disease trajectories of any cancer, and recurrence is quite common,” notes senior and corresponding author Justin Lathia, PhD, Vice Chair of the Department of Cardiovascular & Metabolic Sciences in Cleveland Clinic’s Lerner Research Institute and Co-Director of the Center of Excellence in Brain Tumor Research and Therapeutic Development. “This lethality is due in large part to the emergence of the cancer stem cell phenotype. Because the factors that influence cancer stem cell populations are not clear, we set out to understand the effects of diet on cancer stem cell enrichment.”
The researchers fed mice with glioblastoma an obesity-inducing high-fat diet or a standard diet. They observed that, compared with a standard diet, the high-fat diet was associated with more aggressive disease accompanied by cancer stem cell enrichment and reduced survival.
They then collected tissue samples from the two groups and compared them using advanced analytic and imaging techniques. “Brain tissue from the high-fat group exhibited an increased accumulation of saturated fats, which curtailed hydrogen sulfide production and signaling by as much as 50%,” says Daniel Silver, PhD, a research associate in the Lathia lab and first author on the study. “In the absence of hydrogen sulfide, tumor cell growth was increased; the aggressive, stem-like character of the malignancy was enhanced; and necrotic cell death was nearly eliminated.”
Advertisement
Hydrogen sulfide has been a central focus of research in aging, neurodegeneration and metabolism, but little is currently known about its role in cancer.
Co-investigator Christopher Hine, PhD, also in the Department of Cardiovascular & Metabolic Sciences, is a biology of aging researcher who has spent much of his career studying hydrogen sulfide. In tandem papers published earlier in 2021, he showed that every-other-day fasting improved metabolic, muscular and cognitive fitness in aged preclinical models. According to those findings, dietary restriction increases the production capacity and bioactivity of hydrogen sulfide, which is suspected to confer the metabolic and aging-related benefits.
In the current study, the researchers found that the diet-associated hydrogen sulfide dysfunction ultimately increased proliferation of cancer cells and enabled them to become resistant to treatment. Conversely, stimulating hydrogen sulfide production or administering hydrogen sulfide was found to help kill glioblastoma cells in culture and prevented tumor growth.
“We identified hydrogen sulfide signaling as a possible tumor suppressor for glioblastoma that is highly sensitive to dysregulation by dietary fat,” explains Dr. Hine. “This may have important implications for identifying new therapeutic targets. Our next steps will be to investigate the specific dietary and/or aging-related mechanisms that cause reduced hydrogen sulfide signaling in the brain.”
The study was funded in part by Cleveland Clinic and Case Comprehensive Cancer Center, for which Dr. Lathia serves as co-leader of the Molecular Oncology Program. Dr. Lathia is also scientific director of Cleveland Clinic’s Rose Ella Burkhardt Brain Tumor & Neuro-Oncology Center.
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity
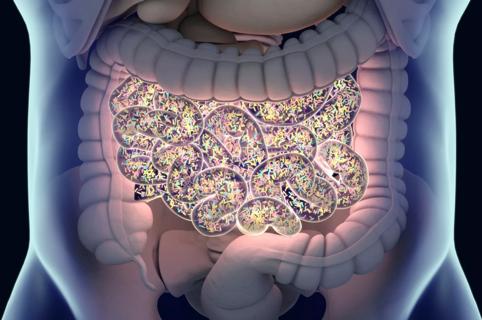
Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time
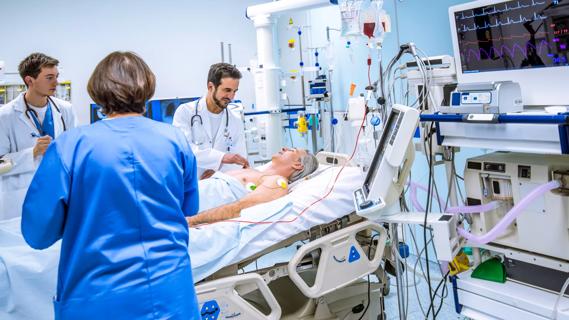
Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology