In vitro model of blood-brain barrier points to potential therapeutic target
Glucocorticoid receptors (GRs) play a significant role in pharmacoresistant epilepsy and represent a potential therapeutic target, suggests an ongoing line of investigation by Cleveland Clinic researchers.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“We believe that dysfunction of the blood-brain barrier (BBB) contributes to resistance to antiepileptic drug therapy in about 25 to 30 percent of epileptic patients with uncontrolled seizures,” says lead researcher Chaitali Ghosh, PhD, Staff Scientist in the Department of Biomedical Engineering, Cleveland Clinic Lerner Research Institute, and Assistant Professor of Molecular Medicine, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University. “So we set out to examine how mechanistic regulation of glucocorticoid receptors in endothelial cells in the human epileptic brain could affect BBB properties. We also aimed to explore the relevance of glucocorticoid receptors to drug penetration across the BBB and to drug metabolism.”
The latest work by Dr. Ghosh’s group, which includes coinvestigators at Lerner Research Institute and clinical colleagues from Cleveland Clinic’s Epilepsy Center, builds on an earlier study by her team (Epilepsia. 2017;58:576-585) that focused on nuclear receptors — e.g., GRs and pregnane X receptors — involved in regulation of cytochrome P450 (CYP450) enzyme expression. They found that the nuclear receptors were co-expressed with CYP450 enzymes, which metabolize a number of medications, including antiepileptic drugs, and contribute to drug resistance in the human epileptic brain (Figure 1).
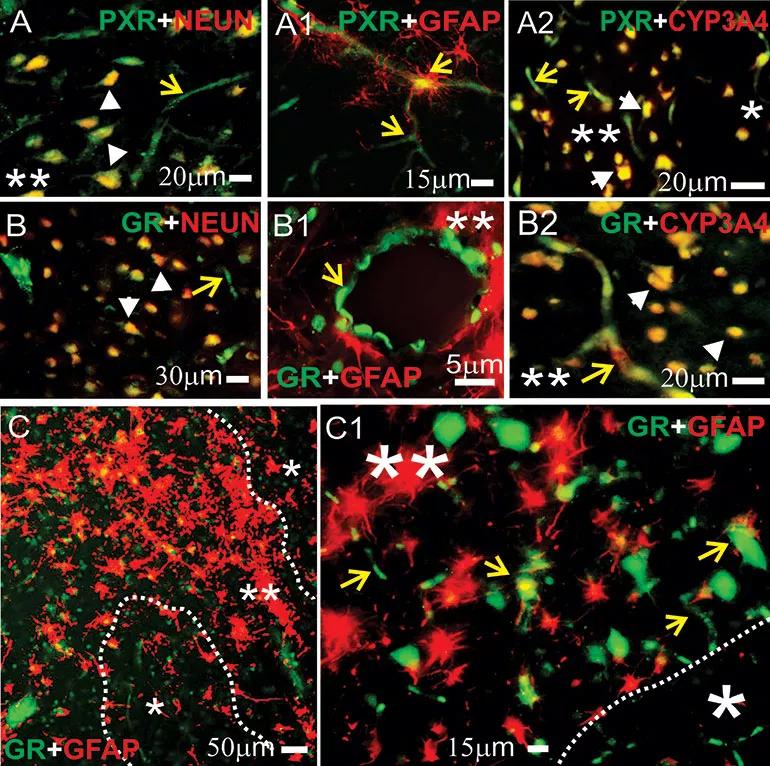
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b42c7bf9-0fbb-414a-825a-150c8a35fdfd/19-NEU-230-ghoshEpilepsia-inset1-770x766_jpg)
Figure 1. Expression of pregnane X receptors (PXRs), glucocorticoid receptor (GRs) and cytochrome P3A4 (CYP3A4) in human epileptic brains. (A-C1) GRs and PXRs were found at the blood-brain barrier interface and in neurons in epileptic brains. Magnification of gliotic regions (**) and GFAP+ staining on a resected human epileptic brain showed increased expression of GRs, PXRs and CYP3A4. This staining pattern was less evident in nongliotic regions (*). Note: Neurons are indicated by white arrowheads and capillaries by yellow arrows. Co-localization was evaluated with neuronal markers (NEUN, panels A-B) and glial markers (GFAP, panels A1-B1 and C-C1). (A-A2) PXR expression was observed at the vascular interface (A-A1, yellow arrows) and in neurons (A and A2). (B-B2) A similar pattern was seen for GR expression across the epileptic brain slices. (A2, B2) Co-localization of GR or PXR staining with CYP3A4 was found in regions with reactive gliosis (**). (C-C1) Representative images of brain samples from patients with temporal lobe epilepsy show GR staining in regions with reactive gliosis (**). Reprinted from Ghosh et al., Epilepsia. 2017;58:576-585.
Advertisement
“We showed that endothelial cells isolated from postsurgical human brain specimens from patients with epilepsy overexpressed both glucocorticoid and pregnane X receptors — as well as CYP450 enzymes —relative to control brain endothelial cells,” Dr. Ghosh explains.
In their latest study (Epilepsia. 2018;59:2049-2060), the researchers found that by downregulating GR expression in endothelial cells of the BBB in human epileptic brain — either by GR silencing or by pharmacologic GR inhibition — they could reduce levels of CYP450 enzymes and drug-efflux transporters such as multidrug resistance protein 1 (MDR1) (Figure 2).
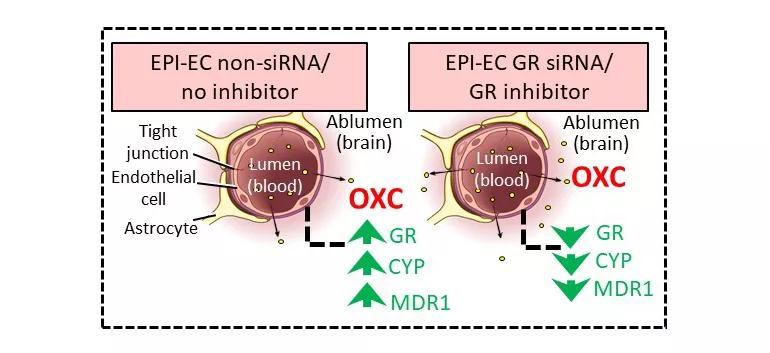
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/53c52d7d-2142-45e0-bdde-4646ce82db5c/19-NEU-230-ghoshEpilepsia-inset2-770x353_jpg)
Figure 2. Schematic representation of improved oxcarbazepine (OXC) penetration across the blood-brain barrier (BBB). The nonsilenced or noninhibited epileptic brain endothelial cell (EPI-EC) condition demonstrated increased drug metabolic potency at the BBB. Conversely, silencing glucocorticoid receptors (GRs) in EPI-ECs resulted in a reduction of OXC metabolism compared with nonsilenced conditions in EPI-ECs. Penetration of OXC across the BBB EPI-EC was thereby improved upon GR silencing or inhibition. CYP = cytochrome P; MDR1 = multidrug resistance protein 1. Reprinted from Ghosh et al., Epilepsia. 2018;59:2049-2060.
Using endothelial cells derived from surgically resected brain specimens from patients with pharmacoresistant epilepsy, the researchers recreated a physiologically relevant, flow-based in vitro BBB model for the study. They used the model to test silencing and inhibition of GRs, which diminished expression of pregnane X receptor, the CYP3A4 enzyme and MDR1.
Advertisement
The findings suggest that GR modulation in epileptic endothelial cells does several things, the investigators noted:
“This latest study indicates that glucocorticoid receptors may play a significant role in expression of pharmacoresistance in epilepsy by way of reduced drug bioavailability to epileptic brain areas,” Dr. Ghosh notes. “We showed that this role depends on downstream regulation of drug-metabolizing enzymes and efflux transporters in endothelial cells at the BBB. This work paves the way toward consideration of the glucocorticoid receptor as a therapeutic target in the endothelium to influence brain vasculature in a way that overcomes the pharmacokinetics behind resistance to antiepileptic medications.”
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity
Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time
Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology