Hsp90 acts as chaperone and stabilizer for globins

New research from Cleveland Clinic has identified a major player in the process by which hemoglobin (Hb) forms and matures; understanding of this process has been elusive until recently.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The new finding allows researchers to better identify potential problems in blood development, according to one of the researchers, Dennis J. Stuehr, PhD, of the Lerner Research Institute’s Department of Pathobiology. It has potential for use in developing treatments for cancer — since some cancer cells utilize Hb differently than red blood cells — and blood diseases such as sickle cell anemia, thalassemia and anemia, he says. The research was published in the Proceedings of the National Academy of Sciences of the USA.
Specifically, Drs. Stuehr, Arnab Ghosh, PhD, and his colleagues found that a protein called heat shock protein 90 (hsp90) is critical in the development of mature, functional hemoglobin, acting as a chaperone for beta and gamma globins. These globins combine with heme to form mature Hb that can deliver oxygen throughout the body. “Hsp90 is a big guy on the block, and there is even a bi-annual conference devoted to its role and related chaperone machinery,” explains Dr. Stuehr.
Previous research in the field had shown that another chaperone protein called alpha hemoglobin stabilizing protein (AHSP) binds to alpha globins during erythropoiesis.
The researchers studied Hb maturation in three cells lines, both erythroid and nonerythroid: the human erythroid leukemia cell line K562 and human erythroid progenitor cells HiDEP-1 and HUDEP-2 during differentiation, and nonerythroid cell lines that express Hb naturally or in a transient manner. They looked specifically for a role for hsp90 as a chaperone.
Advertisement
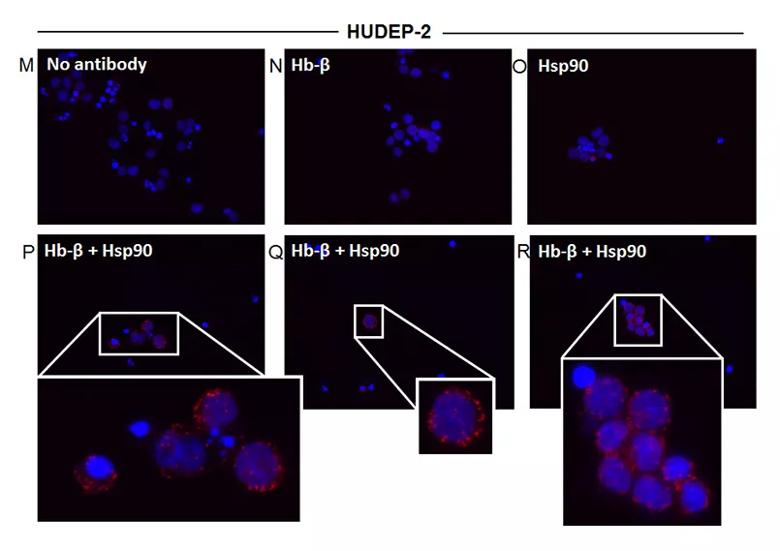
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/cdfe3ed0-bf6b-4aa4-8989-0d3708c644d2/805x-Inset-1-PNAS_jpg)
Distinct Hsp90 and AHSP globin interactions occur in erythroid progenitor cells during red blood cell differentiation. Erythroid progenitor cells HiDEP-1 or HUDEP-2 were induced to differentiate for a 16-d period, cell aliquots were harvested before or during differentiation, and the soluble supernatants were analyzed. (M) Control using no primary antibody, (N) Hb-β antibody alone, (O) Hsp90 antibody alone, and (P–R) PLA images with both Hb-β and hsp90 antibodies with insets showing close-ups. Red dots indicate the location and extent of interaction.
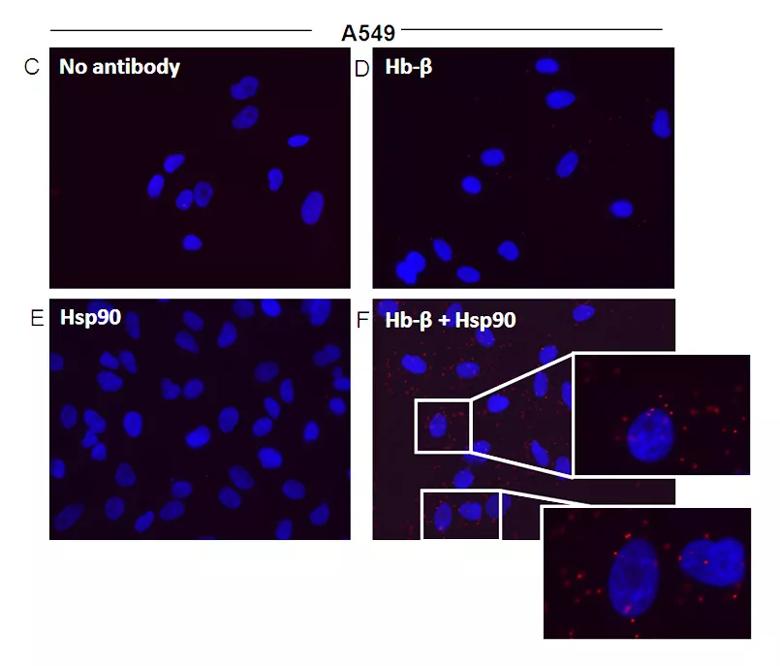
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ab9d1968-a77b-4a38-b2ab-1188f0b0ec8a/805x-Inset-4-PNAS_jpg)
Hsp90-globin associations in nonerythroid cells that naturally express Hb. RAW and A549 cell supernatants were analyzed for expression of Hb, hsp90, and ASHP, and the hsp90-globin associations were determined. The RAW cells were either resting or induced by a 16-h culture with IFN-γ + LPS before lysis. (C–F) Representative PLA images indicating colocalization of hsp90 and Hb-β in A549 cells. (C) PLA controls using no primary antibody, (D) Hb-β antibody alone, (E) Hsp90 antibody alone, (F) PLA images with both Hb-β and hsp90. antibodies with Insets showing close-ups. Red dots indicate the location and
extent of the interaction.
In the immature, heme-free state, hsp90 was found to be essential for heme insertion, globin specific, and a driver of globin maturation during erythropoiesis. “We found that hsp90 binds to immature, heme-free beta and gamma globins in erythroid-like cells, stabilizing the globins and helping to drive their heme-insertion reactions,” reports Dr. Stuehr. “Hsp90 then dissociates from the globins and is replaced by a protein partner, allowing heterotetramer formation to occur with globin alpha.” They found that hsp90 did not associate with mature Hb, however, he says.
Advertisement
Hsp90 was also found to drive globin maturation. In nonerythroid cells. “Our work showed that in situations where there is no AHSP protein, hsp90 can take over for that protein and help mature both alpha and beta globins in nonerythroid cells,” he says, adding, “We answered a paradox as to how without AHSP you can get functional, mature hemoglobin outside of the erythroid system.”
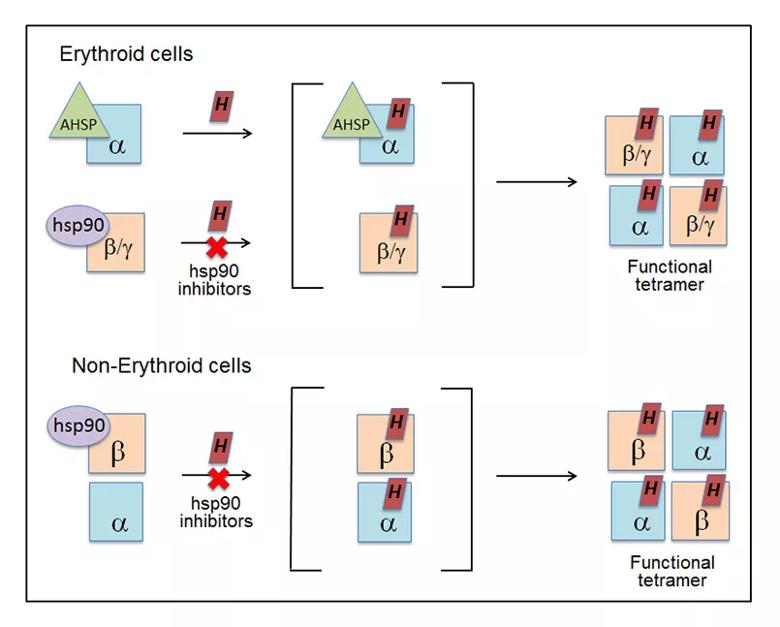
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f4a24ee4-6a0b-4f66-a871-5a3c618bd3fc/805x-Inset-3-PNAS_jpg)
Model for chaperone involvement in Hb maturation. In erythroid cells, the immature, heme-free globins associate with their specific chaperones (AHSP or hsp90). Hsp90 helps drive heme insertion into its globin partner, and after the chaperones dissociate, the heme-replete globins interact to form functional hetero-tetramers. In nonerythroid cells, the heme-free globins associate in a complex with hsp90. In this case, heme insertion into the globins is entirely hsp90-dependent and allows them to form functional tetramers.
Although research is still preliminary, Dr. Stuehr said the potential applications of the Cleveland Clinic hsp90 work are exciting and promising. “For instance, no one has looked at whether this pathway with hsp90 has been compromised under some conditions or in some patients, such as those with blood disorders or cancers,” he says, “and that can now be investigated.”
More immediate implications of the research are for drug development. “Drugs that can block hsp90 in cancer cells are currently being investigated,” he reports, “but it’s been found they can cause negative side effects such as anemia. Our research might allow investigators to target hsp90 inhibitors to the cancer itself so it doesn’t attack other healthy cells in the patient’s body. Ultimately, that could lead to less anemia with use of these drugs. And research already suggests that blocking hemoglobin maturation in certain cancers will inhibit their ability to metastasize. ”
Advertisement
Next on the research agenda, Dr. Stuehr and colleagues intend to search for co-chaperones with hsp90 in the hemoglobin maturation process. “We know hsp90 is not the only protein at work here,” he says, “and we expect to see some familiar faces and some new faces.” They also want to examine how hsp90 interacts with hemoglobin at the molecular and genetic levels, and how it functions normally and in disease conditions that compromise its function.
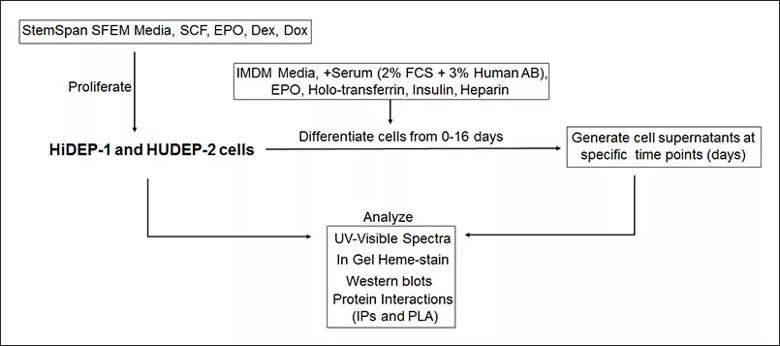
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/032f3213-7c0c-431b-8397-8f0d4c26cbf5/805x-Inset-2-PNAS_jpg)
General methods to monitor Hb maturation in HiDEP-1 and HUDEP-2 cells during proliferation and erythropoietic differentiation. Dex, dexamethasone; Dox, doxicyclin; EPO, erythropoietin; HiDEP-1, human iPS cell-derived erythroid progenitor-1; HUDEP-2, human umbilical cord blood-derived erythroid progenitor-2; IPs, immunoprecipitations; PLA, proximity ligation assay; SCF, stem cell factor.
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity

Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time

Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology