Established lymphoma/leukemia therapy also targets glioma stem cells
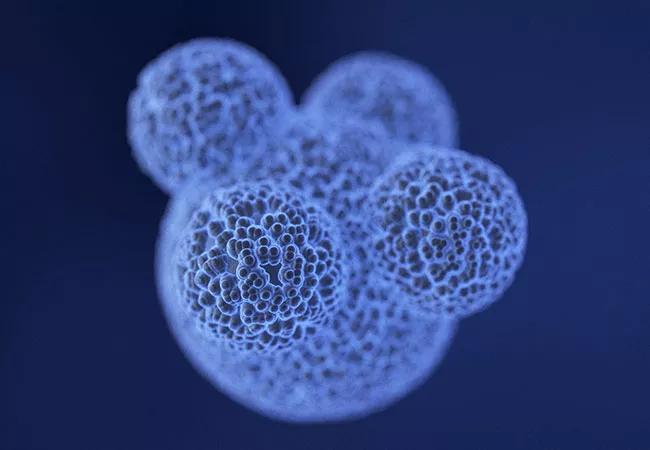
A preclinical study by a Cleveland Clinic-led research team suggests that ibrutinib, a small-molecule drug recently approved by the FDA to treat various forms of lymphoma and leukemia, may also help treat glioblastoma, the most common and lethal type of brain cancer. The promising findings, published May 30 in Science Translational Medicine, offer hope that ibrutinib may ultimately help improve glioblastoma patient outcomes and survival, which currently are exceptionally poor.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The researchers, led by Shideng Bao, PhD, of the Department of Stem Cell Biology and Regenerative Medicine in Cleveland Clinic Lerner Research Institute, found that ibrutinib suppressed tumor growth and increased survival in a preclinical model of glioblastoma. The agent was significantly more effective in slowing tumor growth than the current standard-of-care glioblastoma chemotherapy agent, temozolomide, and extended average survival by more than 10-fold.
Dr. Bao’s team found that ibrutinib works by inhibiting glioma stem cells (GSCs), an especially aggressive type of cancer cell that can self-renew, spread and resist conventional treatments. This aggressiveness makes GSCs particularly lethal and of great interest to researchers as a potential therapeutic target.
In both a preclinical model and cultured human glioblastoma cells, the team found that ibrutinib effectively suppressed GSC-driven tumor growth and potently induced GSC death.
A key characteristic of glioblastoma tumor cells is their ability to evade treatment. Traditional therapies, including chemotherapy and radiation, often work for a while, but the cancer cells eventually stop responding. The highly resistant GSCs enable glioblastoma tumors to rapidly recur. Thus, targeting GSCs is critical for improving glioblastoma treatment.
The researchers discovered in their preclinical (mouse) model that combining radiation therapy with GSC-targeting ibrutinib helped overcome this lethal resistance. Combination therapy overcame therapeutic resistance and extended lifespan more effectively than either radiation or ibrutinib treatment alone.
Advertisement
Dr. Bao’s earlier work demonstrated that GSCs promote glioblastoma resistance to radiation therapy (Nature, 2006). He and colleagues later found that GSCs have high levels of the protein known as bone marrow and X-linked (BMX) nonreceptor tyrosine kinase. They showed that BMX activates the STAT3 molecule (signal transducer and activator of transcription 3) and that “turning on” STAT3 enables GSCs to replicate, spread and promote GSC-driven tumor growth (Cancer Cell, 2011).
In the present study, the researchers found that ibrutinib effectively inhibited BMX activity and thus interfered in the activation of STAT3. Fewer active BMX molecules resulted in fewer activated STAT3 molecules and, therefore, reduced tumor growth and GSC-driven resistance.
While additional research is critical to understand ibrutinib’s effects in patients with glioblastoma, these preclinical findings are highly promising. Since ibrutinib, which is orally administered, is already FDA-approved for use in humans, Dr. Bao says clinical trials of ibrutinib as a treatment for glioblastoma — either alone or in combination with current therapies — are not far off.
The study was supported by grants to Dr. Bao from the National Cancer Institute and National Institute of Neurological Disorders and Stroke as well as grants to Dr. Bao’s collaborator, Xiu-Wu Bian, from the National Key Research and Development Program of China.
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity
Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time
Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology